Hemostasis and Coagulation
Biology of Hemostasis
- Vasoconstriction
- initial vascular response to injury
- local contraction of vascular smooth muscle is a reflex response to various stimuli
- local mediators augment vasoconstriction: thromboxane, endothelin, serotonin, bradykinin
- Platelet Plug Formation
- platelets are 2-μm fragments of megakaryocytes with a lifespan of 7 - 9 days
- injury to the intima exposes subendothelial collagen
- subendothelial von Willebrand’s factor (VWF) binds to the exposed collagen
- the platelet has a receptor for the bound VWF
- VWF serves as a bridge between subendothelial tissue and the platelet
- receptor binding activates the platelet, resulting in a release reaction (degranulation)
- release reaction results in the recruitment of additional platelets (aggregation), mediated
primarily by ADP and serotonin
- degranulation also releases thromboxane, a potent vasoconstrictor and platelet aggregator
- thromboxane is converted from prostaglandin G2 by cyclooxygenase - this step is irreversibly
inhibited by aspirin
- activated platelets express receptors which bind fibrinogen, which in turn binds other activated
platelets together in an aggregate, forming a platelet plug
- aggregation of platelets does not occur in the absence of fibrinogen
- surface of the activated platelet plug is a major substrate for the intrinsic coagulation cascade
- Coagulation Cascade
- results in the formation of insoluble fibrin, which stabilizes the platelet plug
- consists of a series of stages in which circulating proenzymes are converted in sequence to
activated proteases
- all the procoagulants, except VWF, are produced by the liver
- factors II, VII, IX, and X are vitamin K dependent
- without vitamin K, these proteins do not undergo gamma-carboxylation and are thus inactive
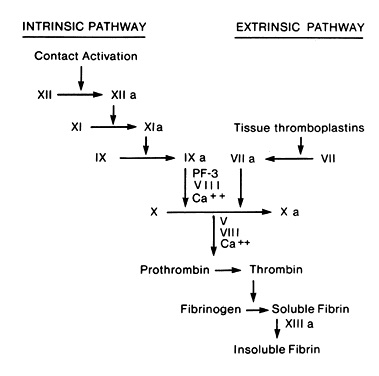
- Extrinsic Pathway
- initiated by tissue lipoprotein (thromboplastin - extrinsic to blood)
- factor VII is activated by thromboplastin and Ca++
- activated factor VII activates factor X
- causes large amounts of clot to be formed in seconds
- monitored by the prothrombin time (or INR)
- Intrinsic Pathway
- initiated by phospholipids intrinsic to blood
- requires several minutes to form a clot
- factor XII is activated by binding to subendothelial collagen
- prekallikrein and high-molecular-weight kininogen amplify this contact phase
- activated factor XII activates factor XI
- activated factor XI activates factor IX
- activated factor IX acts with factor VIII, phospholipids from the injured platelets,
and calcium to activate factor X
- monitored by the activated partial thromboplastin time (PTT)
- Final Common Pathway
- activated factor X converts prothrombin to thrombin
- this process is accelerated by activated factor V, tissue lipoproteins, platelet surface
phospholipids, and Ca++
- thrombin cleaves the fibrinogen molecule into fibrin
- fibrin polymerizes both side-to-side and end-to-end, resulting in a latticework
- cross-linking of fibrin is catalyzed by activated factor XIII
Control of Coagulation
- clot must not form beyond the site of injury
- Blood Flow
- most important control is the continued rapid flow of blood, which carries away thrombin,
procoagulants, products of platelet activation
- Endothelium
- has a negatively charged surface, which repels clotting factors and platelets
- if neither factor XII nor platelets are activated, coagulation cannot be initiated
- intact endothelium synthesizes prostacyclin, which inhibits platelet aggregation
- Circulating Anticoagulants
- Anti-thrombin III
- when combined with thrombin, blocks the enzymatic activity of thrombin on fibrinogen
- neutralizes all the procoagulant proteases
- activity is greatly increased by heparin
- Protein C
- activated by thrombin
- reduces thrombin formation by inactivating factors V and VIII
- Vitamin K dependent
- Protein S
- in conjunction with Protein C, activates plasminogen
- Vitamin K Dependent
- Fibrinolysis
- natural process directed at maintaining the patency of blood vessels by lysis of fibrin deposits
- dependent on the enzyme plasmin, which is derived from plasminogen
- plasminogen is preferentially absorbed onto fibrin deposits, where it is converted to plasmin by
thrombin, activated factor XII, and tissue plasminogen activator (tPA)
- plasmin breaks down fibrin, as well as many of the clotting factors found in blood
- fibrinolysis can be blocked by epsilon-aminocaproic acid or tranexamic acid
Assessment of Hemostasis and Coagulation
- History and Physical
- most important assessment
- questions about past surgical or dental history should detect any prior untoward bleeding
- a family history should detect any hereditary defects
- review medications for oral anticoagulants, aspirin, NSAIDs
- complete medical history should detect liver or renal dysfunction
- Platelet Tests
- Platelet Count
- thrombocytopenia is the most common abnormality of hemostasis in surgical patients
- spontaneous bleeding only rarely occurs when the platelet count > 40,000
- platelet count > 50,000 is usually adequate for hemostasis after surgery or trauma
- Bleeding Time
- assesses both the interaction between the platelets and damaged blood vessel and the
formation of the platelet plug
- will be abnormal in patients with thrombocytopenia, qualitative platelet disorders,
von Willebrand’s disease
- aspirin taken within one week will affect the results
- has largely been replaced by more convenient and standardized tests such as the platelet
function analyzer test (PFA-100)
- Coagulation Tests
- Prothrombin Time (PT)
- assesses the functional capacity of the extrinsic system (factor VII) and the final common
pathway (factors X and V, prothrombin, fibrinogen)
- used to monitor patients on Coumadin, and results are reported as an international
normalized ratio (INR)
- Partial Thromboplastin Time (PTT)
- measures the functional capacity of the intrinsic system (factors XII, XI, IX, VIII) and the
final common pathway
- useful for identifying hemophilia A (factor VIII deficiency), hemophilia B
(factor IX deficiency), hemophilia C (factor XI deficiency)
- heparin blocks the intrinsic system and results in a prolonged PTT
- also used to monitor the effects of the parenteral direct thrombin inhibitors argatroban, bivalirudin, and
lepirudin
- Thrombin Time
- measures the conversion of fibrinogen to fibrin
- very sensitive to heparin and direct thrombin inhibitors
- Fibrinogen Assays
- sensitive test of fibrinogen function
- significantly less affected by the presence of heparin or direct thrombin inhibitors than
the thrombin time test
- Factor Assays
- direct immunoassays for most clotting factors are now available
- Tests of Fibrinolysis
- D-Dimer Assay
- D-dimers are fragments of cross-linked fibrin that are produced by lysis of a fibrin clot
- marker of clot formation
- sensitive test for the presence of DIC and acute thrombosis
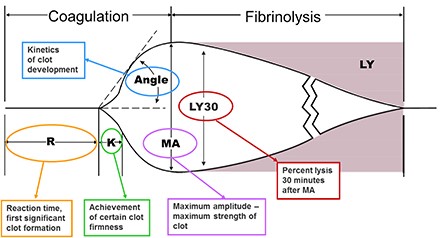
- Thromboelastogram (TEG)
- global test of platelets, coagulation, and fibrinolytic function
- particularly useful for detecting hyperfibrinolysis during liver transplantation and cardiac
surgery
- Interpretation of TEG
- R Value
- time it takes for clot formation to start
- measures coagulation factor activity
- prolonged R value could be treated with FFP
- K Value
- time from the end of R until the clot reaches 20 mm
- clot amplification phase
- represents thrombin’s ability to cleave fibrinogen into fibrin
- elevated with hypofibrinogenemia
- Alpha Angle
- assesses rate of clot formation and fibrin crosslinking
- dependent on fibrinogen
- Maximum Amplitude (MA)
- represents the strength of the final clot
- dependent on platelets (80%) and fibrin (20%)
- will be decreased in patients taking ASA or Plavix
- LY30
- percent clot lysis at 30 minutes
- assesses the fibrinolytic phase
Congenital Hemostatic Defects
- Factor VIII Deficiency (Hemophilia A)
- Inheritance
- disease of males
- sex-linked recessive trait that occurs in 1 in 5000 male births
- spontaneous mutations occur in 20% of cases
- Clinical Manifestations
- severity of clinical manifestations is related to the degree of deficiency of factor VIII
- spontaneous bleeding is rare if the patient has 5% of normal factor VIII activity
- spontaneous joint and soft tissue bleeding are the rule if the patient has < 1% factor VIII
activity
- patients will have a prolonged PTT
- diagnosis is confirmed with a factor VIII assay
- Replacement Therapy
- recombinant (preferred) or plasma-derived factor VIII concentrates are the primary treatment
- half-life of factor VIII is 8 to 12 hours
- for major surgery, levels of 80 to 100 percent should be obtained preoperatively and
maintained for 3 days
- levels should be maintained > 50% for the next 10 - 14 days
- in mild hemophiliacs, DDAVP increases factor VIII activity and can be used in patients
undergoing minor surgery
- Factor IX Deficiency (Hemophilia B)
- clinically indistinguishable from factor VIII deficiency
- X-linked recessive mode of inheritance
- diagnosis is made with factor IX assay
- like factor VIII deficiency, factor IX deficiency can occur in mild, moderate, and severe forms
according to the level of factor IX activity in the blood
- patients have a prolonged PTT
- replacement therapy is with factor IX concentrates
- duration of therapy is similar to patients with factor VIII deficiency
- von Willebrand’s Disease
- autosomal dominant transmission
- most common congenital bleeding disorder
- vWF is required for platelet binding to subendothelial collagen
- clinically, mucosal bleeding (epistaxis, gum bleeding, menorrhagia) predominates
- diagnosis is made by the von Willebrand antigen assay
- most patients with mild disease (types I and II) can be treated with DDAVP, which causes release of preformed stores
of von Willebrand factor
- patients with severe disease (type III) should be treated with von Willebrand-containing factor VIII concentrates
- cryoprecipitate should be avoided because of its risk of disease transmission
- aspirin must be avoided 10 days before an elective procedure
- Congenital Platelet Disorders
- Glanzmann Thrombasthenia
- autosomal recessive disorder
- platelet glycoprotein complex (IIb/IIIa) is missing or dysfunctional
- normal platelet count
- results in poor platelet aggregation and mucocutaneous bleeding
- treatment is with platelet transfusions
- Bernard-Soulier Syndrome
- autosomal recessive deficiency in glycoprotein (GP) Ib, resulting in a defect in the vWF receptor
- prevents platelet linking to collagen
- treatment is with platelet transfusions
- Factor V Leiden
- most common inherited thrombophilia or hypercoagulable disorder
- autosomal dominant mutation of factor V impairing cleavage by protein C
- most common clinical manifestation is VTE and treatment is anticoagulation
Acquired Hemostatic Defects
- Platelet Abnormalities
- Thrombocytopenia
- most common abnormality of hemostasis that results in bleeding in a surgical patient
- results from a variety of disease processes:
- ITP, TTP, lupus
- hypersplenism (splenomegaly, portal hypertension)
- chemotherapy
- massive transfusions
- drugs (heparin, histamine blockers)
- viral infection
- for an elective operation, a platelet count > 50,000 requires no specific therapy
- one unit of platelets will raise the platelet count by 10,000
- Impaired Platelet Function
- Anti-platelet Drugs
- aspirin, clopidogrel (Plavix), and prasugrel (Effient) all irreversibly inhibit
platelet function
- if possible, the drug should be stopped 5 – 7 days before an elective procedure
- in emergency cases, platelet transfusions may be necessary
- Uremia
- the platelet dysfunction of chronic kidney disease can often be corrected by
dialysis or the administration of DDAVP
- Acquired Hypofibrinogenemia
- Disseminated Intravascular Coagulation (DIC)
- caused by the introduction of thromboplastic materials into the circulation, resulting in
excessive thrombin generation and diffuse formation of microthrombi
- end result is the formation of diffuse microthrombi with consumption of platelets,
coagulation factors, and fibrinogen
- diffuse hemorrhage usually dominates the clinical picture
- many disease processes may activate the coagulation system: sepsis, trauma, burns,
obstetric disasters, snakebites
- diagnosed by the appropriate clinical setting and lab values (↑ PT, ↑PTT, ↓ platelets,
↓ fibrinogen, ↑ fibrin split products, ↑ D-dimer)
- treatment is directed at the causative medical or surgical problem
- also important to maintain capillary flow with IV fluids
- if there is active bleeding, hemostatic factors should be replaced with fresh frozen plasma
and cryoprecipitate
- most studies show that heparin is not helpful in acute forms of DIC
- Fibrinolysis
- results from release of excessive plasminogen activator
- prostate operations can cause the release of urokinase into the circulation
- also seen in patients on extracorporeal bypass
- treatment is with epsilon-aminocaproic acid (Amicar), which interferes with fibrinolysis by
inhibiting plasminogen activation
- Liver Failure
- hepatic failure is associated with coagulopathy since nearly all the clotting factors are
synthesized in the liver
- cirrhosis is also associated with thrombocytopenia (hypersplenism)
- FFP and cryoprecipitate are the mainstays of treatment for liver coagulopathy
- platelet transfusions will often be necessary before invasive procedures, but their effect is only
for several hours
- proteins C and S, as well as anti-thrombin III, are also made in the liver and may account for a
hypercoagulable state in failing livers
- Hypercoagulable Disorders
- Heparin Induced Thrombocytopenia (HIT)
- heparin-associated antiplatelet antibodies develop after exposure to heparin products
- clinically, can result in venous or arterial thromboembolism
- suspect if platelets <50,000 or decreased >50% and confirm with ELISA or serotonin release assay (SRA)
- treatment is to discontinue all heparin products and start a direct thrombin inhibitor
- Antiphospholipid Antibody Syndrome
- venous or arterial thromboembolism associated with laboratory evidence of antiphospholipid antibodies
(anticardiolipin or anti-beta 2 glycoprotein I antibodies)
- can occur as a primary condition or associated with autoimmune disease or pregnancy
- may present with a prolonged PTT or thrombocytopenia
- treatment is anticoagulation
- Antithrombin III Deficiency
- can be acquired or congenital
- associated with increased risk of VTE
- diagnosed with decreased levels of ATIII on laboratory evaluation
- VTE cannot be treated with heparin; use a direct thrombin inhibitor, warfarin, or direct oral anticoagulant
Antiplatelet Drugs
- Aspirin
- irreversible inhibitor
- long duration of action (7 days)
- can be safely continued in low-risk patients
- Clopidogrel (Plavix)
- most common use is in prevention of coronary or vascular stent thrombosis
- often used along with low-dose aspirin (81 mg)
- elective surgery should be delayed for at least the minimum recommended duration for each stent type
- if emergent surgery is required, 24-hour interventional cardiology should be available
- if the risk of bleeding is high, Plavix should be stopped 5 – 7 days before surgery
- low-dose aspirin can often be continued
- should be resumed as early as possible in the post-op period
Anticoagulant Drugs
- Heparin (Unfractionated)
- naturally occurring glucosaminoglycan
- forms a complex with anti-thrombin III, which inactivates thrombin as well as factors XII, IX, X, XI
- half-life is about 1 hour
- best monitored by the PTT
- clinical situations associated with bleeding include cardiac bypass surgery, hemodialysis, and
patients being treated for PE or DVT
- effects may be reversed by protamine: 1 milligram of protamine neutralizes 100 units of heparin
- continuous infusion technique reduces the risk of spontaneous bleeding
- can be given SQ for DVT prophylaxis
- thrombocytopenia can be a limiting factor (HIT)
- Low Molecular Weight Heparin (LMWH)
- Lovenox (enoxaparin), Fragmin (daleparin)
- binds to and activates antithrombin III
- inhibits factor Xa, but not thrombin
- may be used prophylactically or therapeutically
- clinical bleeding usually occurs in patients being treated for DVT or PE who develop worsening
renal function
- can’t be monitored by the PTT
- protamine reverses a variable amount of LMWH activity (Lovenox 54%)
- causes less HIT than heparin
- SQ use only
- Fondaparinux (Arixtra)
- IV agent
- indirect inhibitor of Xa; does not inhibit thrombin
- used in acute coronary syndromes and the treatment of VTE
- no lab monitoring available
- Warfarin (Coumadin)
- is used for chronic anticoagulation (atrial fibrillation, mechanical cardiac valves, VTE)
- inhibits the activation of the vitamin K-dependent factors (II, VII, IX, X)
- numerous drug – drug interactions and variable bioavailability makes dosing difficult and
unpredictable
- factor VII function is the most sensitive indicator of the warfarin effect, since it has the
shortest half-life (2 - 4 hrs)
- monitored by the prothrombin time/INR
- protein C and protein S are also vitamin K-dependent anticoagulants
- if these proteins are affected to a greater degree than the clotting factors, then a
hypercoagulable state can arise
- rapid reversal can be achieved with IV vitamin K and prothrombin complex concentrate
- FFP reversal is slow and requires large volumes
- oral vitamin K will reverse Coumadin within 24 hours
- Direct Factor Xa Inhibitors
- bind directly to factor Xa, rather than enhancing the activity of antithrombin, as is done by heparin
- only oral agents are available
- most common indication is for stroke prevention in patients with atrial fibrillation
- other uses include treatment of venous thromboembolism, ischemic heart disease,
and heparin-induced thrombocytopenia
- contraindicated in patients with prosthetic heart valves, severe kidney or liver disease, or pregnancy
- very predictable bioavailability, so lab monitoring of drug levels is not required
- most agents have a half-life of 12 hours, but this may be prolonged in older patients
- rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban are the most common agents in use
- andexanet alfa is now available as a reversal agent
- Direct Thrombin Inhibitors
- prevent thrombin from converting fibrinogen to fibrin
- IV Agents
- bivalirudin, argatroban
- short half-lives
- may be monitored by the PTT
- primarily used in patients undergoing percutaneous coronary interventions
- may also be used in patients with heparin-induced thrombocytopenia
- no reversal agents available
- Dabigatran (Pradaxa)
- oral agent
- used in stroke prevention in patients with atrial fibrillation, in prevention and management of VTE,
and in ischemic heart disease
- half-life of 12 - 17 hours
- no routine laboratory monitoring is necessary
- renally excreted, so dialysis can be used in cases of life-threatening bleeding
- Idarucizumab is available as a reversal agent
- Thrombolytics
- activate plasminogen to facilitate clot breakdown
- tPA (tissue plasminogen activator) is most commonly used, but streptokinase and urokinase are also available
- keep fibrinogen level > 100 mg/dL to minimize risk of bleeding
Perioperative Management of Patients Receiving Anticoagulants
- a balance between reducing the risk of thromboembolism and preventing excessive bleeding must be reached for
each patient
- Estimating Thromboembolic Risk
- conditions that increase thromboembolic risk include atrial fibrillation, prosthetic heart valves,
recent venous or arterial thromboembolism, coronary artery disease, and stroke
- clinician must estimate the risk of a thromboembolic event occurring if the antithrombotic agent is
discontinued perioperatively
- mortality rate for mechanical heart valve thrombosis is 17.5%, and for ischemic stroke, 37%
- mortality from major bleeding while on antithrombotic agents is ~ 6% - 10%
- Nonvalvular Atrial Fibrillation
- most common indication for chronic anticoagulation
- individual patient risk factors determine thromboembolic risk
- important risk factors include congestive heart failure, hypertension, age ≥ 75,
diabetes, previous stroke/TIA
- points can be assigned to risk factors to calculate an overall thrombotic risk score (CHADS2)
- low risk patients have a < 5% annual risk of VTE while on anticoagulation; moderate risk
patients have a 5% - 10% annual risk; and high risk patients have > 10% annual risk
- the VTE risk can be used to determine whether heparin bridging is indicated preoperatively
- Prosthetic Heart Valves
- Mechanical Valves
- patients with bileaflet aortic valves without previous stroke or atrial fibrillation
have a low annual risk of thromboembolism (< 5%) while anticoagulated
- aortic valve patients with atrial fibrillation have moderate risk (5% - 10%)
- high risk factors (> 10% annual risk) include patients with mitral valve prostheses,
caged-ball or tilting-disk aortic valves, or previous thromboembolic events
- Bioprosthetic Valves
- porcine or bovine valves do not require long-term anticoagulation (3 – 6 months)
- all percutaneous aortic valves are bioprosthetic
- elective operations should be delayed for 3 – 6 months after implantation
- for emergent operations in the 3 – 6 month window, coumadin can be stopped without
any heparin bridging
- Venous Thromboembolism
- thromboembolism within 3 months is a high risk factor for recurrent thromboembolism if
anticoagulation is stopped
- after 3 months of anticoagulation, the annual risk of recurrent VTE is 15%
- elective surgery should be deferred for at least 3 months after a VTE
- additional risk factors such as cancer or inherited thrombophilias will also need to be
considered
- Coronary Artery Disease
- dual antiplatelet therapy is prescribed after coronary stent placement, and discontinuation
is a strong risk factor for stent thrombosis
- elective surgery should be delayed for > 14 days after balloon angioplasty, 30 days after
bare-metal stent placement, and 1 year after drug-eluting stent placement
- for emergency procedures, the cardiologist should be involved in risk/benefit decisions
regarding stopping the antiplatelet medications
- Stroke
- low-dose aspirin and/or Plavix is recommended for the treatment of acute stroke and
secondary prevention after ischemic stroke or TIA
- stroke patients also have an increased risk for cardiovascular complications
- the elevated risk for cardiac complications plateaus at 9 months after an ischemic stroke;
therefore, elective surgery should be deferred for at least 9 months, if possible
- Estimating Procedure Bleeding Risk
- bleeding risk is determined based on the invasiveness of the procedure and the sequelae of bleeding
if it occurs
- spinal anesthesia, cardiac, vascular, and intracranial procedures are especially risky
- low risk procedures (skin surgery, hernias, elective cholecystectomy) do not usually require
discontinuation of antithrombotic agents
- patient comorbidities (liver, kidney disease) may also contribute to bleeding risk
- the surgeon is responsible for assessing bleeding risk based on the patients individual anatomy,
pathology, risk factors, and their own experience with the procedure
- Deciding Whether to Interrupt Anticoagulation
- decisions must be made on a case by case basis – no scoring system can substitute for clinical
judgement
- in general, if surgical bleeding risk is high, anticoagulants must be discontinued
- the period off anticoagulation should be as short as possible
- in lower risk cases, anticoagulants should usually be continued
- Timing of Anticoagulant Interruption
- Coumadin
- discontinue 5 days before surgery
- check INR on the day before surgery
- surgery when INR < 1.5
- low dose vitamin K may be necessary
- high risk patients (recent stroke, mechanical valve) may require a bridging agent
started 3 days before surgery
- Coumadin can usually be resumed 12 – 24 hours after surgery
- it takes 5 – 10 days to get INR > 2.0, so a heparin bridging agent may be required
- Direct Oral Anticoagulants (DOACs)
- Dabigatran (Pradaxa), Apixaban (Eliquis), Rivaroxaban (Xarelto)
- for minimal bleeding risk, discontinue on the day of surgery only
- for low/moderate bleeding risk, discontinue the day before surgery and restart
the day after surgery
- for high bleeding risk, discontinue 2 days before surgery and restart 2 days after surgery
- if the GI tract is unavailable post-surgery, bridging with LMWH is necessary
- Bridging Anticoagulation
- requires using a short-acting agent (LMWH) during the interruption of a long-acting agent (Coumadin or DOACs)
- intent is to minimize the time the patient is not anticoagulated
- can cause increased risk of bleeding
- bridging can be used pre-op, post-op, or both
- indications for bridging include:
- embolic stroke or arterial embolus within previous 12 weeks
- mechanical mitral valve, mechanical aortic valve and additional stroke risk factors
- atrial fibrillation and high risk of stroke
- venous thromboembolism within the last 12 weeks
- recent coronary stenting
- LMWH is discontinued 24 hours before surgery; IV heparin is discontinued several hours before surgery
- post-op bridging is restarted once hemostasis is assured
- resumption of bridging too soon can result in increased risk of major bleeding
- Coumadin is generally restarted on the same day as the heparin
References
- Schwartz, 10th ed., pgs 85 - 96
- Simmons and Steed, pgs 30 – 37
- UpToDate. Perioperative Management of Patients Receiving Anticoagulants. James D. Douketis, MD, FRCPC, FACP, FCCP,
Gregory YH Lip, MD, FRCPE, FESC, FACC. July 2021. Pgs 1 – 34
- UpToDate. Direct Oral anticoagulants (DOACs) and Parenteral Direct-Acting Anticoagulants: Dosing and Adverse Effects.
Lawrence LK Leung, MD. July 2021. Pgs. 1 – 72
- Cameron, 13th ed., pgs 1253 – 1255, 1457 – 1466
- Hornor, et al., American College of Surgeons’ Guidelines for the Perioperative Management of
Antithrombotic Medication. November 2018: 227, no. 5. Pgs 521 - 536
- Sabiston, 20th ed., pgs 294 – 296, 567
- UpToDate. Congenital and acquired disorders of platelet function. Stephen Coutre, MD. Aug 2021
- UpToDate. Factor V Leiden and activated Protein C resistance. Kenneth A Bauer, MD. Feb 2021.UpToDate.
Diagnosis of antiphospholipid syndrome. Doruk Erkan, MD, MPH, Thomas L Ortel, MD, PhD. Feb 2020
- UpToDate. Antithrombin deficiency. Kenneth A Bauer, MD

