Wound Healing and Wound Management
Phases of Wound Healing
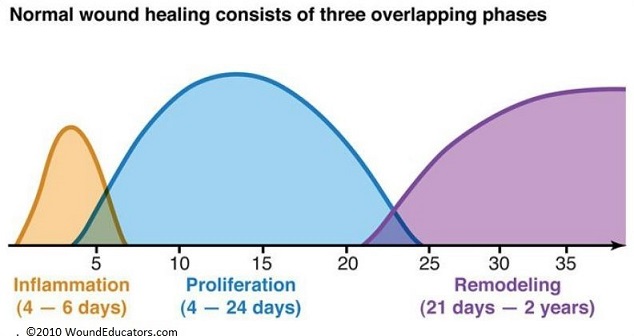
- Hemostasis and Inflammation
- exposure of blood to subendothelial collagen is the initiating factor in wound healing
- Hemostasis
- Vascular Response
- biphasic response (1) vasoconstriction → (2) vasodilation
- vasoconstriction, which serves a hemostatic function, lasts only several minutes
- vasoconstriction lasts only several minutes, and is followed by vasodilation
- vasodilation is initially mediated by histamine, which is produced by platelets,
mast cells, basophils
- vasodilation → ↑ vascular permeability, permitting blood-borne factors to enter the wound
- Platelet Response
- forms a hemostatic plug and initiates coagulation
- produces multiple essential cytokines, which modulate most of the subsequent wound
healing events
- platelet-derived growth factor (PDGF)
- transforming growth factor-alpha (TGF-α)
- transforming growth factor-beta (TGF-β)
- Coagulation Response
- intrinsic and extrinsic coagulation cascades serve both hemostatic and
inflammatory functions
- generation of fibrin provides a scaffold for the migration of inflammatory and
mesenchymal cells
- thrombin contributes to the increased vascular permeability seen after injury,
facilitates the extravascular migration of inflammatory cells, and may have a role
in epithelialization and angiogenesis
- Cellular Migration into the Wound
- Neutrophils
- migrate in response to the chemotactic factors released during injury and
inflammation (bacterial products, C5a, PGE2, PDGF, IL-1, IL-8, TNF-α)
- presence in the wound peaks at 24 – 48 hours
- primary functions are phagocytic and microbicidal
- produce collagenases, which degrade ground substance and matrix in the early phase
of wound healing
- do not have a role in collagen synthesis, thus are not necessary for wound healing
- however, there is an increased rate of infection when neutrophils are not present
- Macrophages
- replace the neutrophil as the dominant cell in the wound by the third or fourth day
- essential for normal wound healing - main cell regulating the proliferative phase
- main cell regulating the proliferative phases of wound healing
- play an active role in wound debridement and connective tissue matrix remodeling
- produce many cytokines that regulate other cellular activities
(IL-1, IL-6, TNF-α, TGF-β, VEGF)
- stimulate angiogenesis and collagen synthesis
- T Lymphocytes
- peak in the wound at one-week post injury
- essential to wound healing, but exact role is unclear
- play an active role in the modulation of the wound environment
- may down-regulate fibroblast collagen synthesis
- Proliferation Phase
- Fibroblast Migration and Proliferation
- by the 4th day, the dominant cell in the wound is the fibroblast
- strongest chemotactic factor for fibroblasts is PDGF
- fibronectin provides a physical pathway along which the fibroblast can migrate
- activation of fibroblasts is mediated by cytokines released by wound macrophages
- Angiogenesis
- essential to successful wound healing
- stimulated by elevated lactate levels, acidic pH, and tissue hypoxia
- all angiogenesis begins with the endothelial cell
- new vessels originate as capillaries which sprout from the sides of small vessels in
response to local angiogenic factors
- cytokines (VEGF, TNF-α, TGF-β) stimulate endothelial cell migration and proliferation
- most of these cytokines are derived from macrophages
- Matrix Formation
- as fibroblasts invade the wound, they manufacture additional new matrices, including
glycoproteins, structural proteins, and adhesive proteins
- Proteoglycans
- hyaluronic acid dominates the early wound matrix
- chondroitan sulfate, dermatan sulfate, heparin sulfate are other common
proteoglycans
- assembly of collagen into fibrils and fibers is dependent upon the structure
provided by proteoglycans
- Collagens
- family of fibrous proteins secreted by the fibroblast
- at least 18 types, but types I and III are most important for wound repair
- contains the amino acids hydroxylysine and hydroxyproline
- vitamin C is necessary for collagen production
- consists of 3 polypeptide chains, each chain twisted into a right-handed helix
- collagen is secreted into the extracellular space as procollagen
- procollagen is cleaved to tropocollagen
- tropocollagen molecules then aggregate into fibrils
- fibrils are cross-linked in the extracellular matrix and aggregate to form collagen
fibers
- the macrophage is the key cell regulating collagen production by the fibroblast,
presumably by secreting growth factors
- collagen synthesis is highly dependent on systemic factors: adequate oxygen supply,
sufficient nutrients, vitamins, trace metals, lack of infection
- Elastin
- secreted into the extracellular matrix as random coils, allowing the network to
stretch and recoil
- Fibronectin
- attachment protein
- aids in cellular attachment
- modulates the migration of various cell types into the wound
- chemotactic for fibroblasts
- Wound Contraction
- begins 4 to 5 days after wounding
- represents the centripetal advancement of the wound edge towards the center of the wound
- myofibroblast appears to be the responsible cell - it contains actin and myosin filaments
and appears when contraction starts and disappears when contraction is complete
- contraction of a large wound across a joint surface can lead to a contracture
- skin-grafting is one of the most effective methods of controlling contraction; full-thickness
grafts are more effective than partial-thickness grafts
- Epithelialization
- involves 2 major phenomena: migration and mitosis
- in the first 24 hours after wounding, thickening of the basal layer occurs
- basal cells then detach from the underlying basement membrane and migrate into the wound
- cells migrate as a sheet over the collagen-fibronectin wound surface
- migrating cells originate from the margins of the wound and skin appendages (hair follicles,
sebaceous glands)
- epithelial cell proliferation contributes new cells to the advancing epithelial cell monolayer
- cells migrate until they reach cells migrating from a different direction
(contact inhibition)
- cellular proliferation continues until a multi-layered epidermis is re-established
- migration and proliferation is stimulated by epidermal growth factor (EGF)
- regenerated epithelium has several important differences from normal epithelium:
- fewer basal cells
- an abnormal dermal-epidermal junction (no rete pegs)
- epithelium thicker at the wound edge than in the midportion
- Wound Remodeling and Maturation
- cell and matrix changes in the wound continue long after completion of epithelialization
- after 21 days, collagen content of the wound becomes stable
- bursting strength of the wound is only 15% of normal skin at 21 days
- wound strength increases without further increases in the wound’s collagen content
- process of scar remodeling increases the wound’s strength by greatly increasing the number of
cross-links between collagen fibers
- by 6 weeks after wounding, the scar reaches 80 - 90 % of its eventual strength
- bursting strength of scar never reaches that of unwounded skin
- during terminal wound healing, a continual turnover of collagen molecules occurs as old collagen is
broken down and new collagen is synthesized
- collagen fibers become more linearly organized along stress lines
Factors Affecting Wound Healing
- Local Factors
- Infection
- requires > 105 bacteria/gram of tissue
- foreign bodies, hematomas, necrotic tissue increase the risk of wound infection
- impaired circulation and radiation further increase the risk
- systemic diseases such as diabetes, AIDS, uremia, and cancer are associated with increased
risk of wound infection
- best treatment is prevention
- prevention requires meticulous technique, judicious use of perioperative antibiotics, and
good judgement as to which wounds should be closed primarily
- Hypoxia
- delivery of oxygen to healing tissues is critical for all aspects of wound healing
- adequate tissue oxygenation requires an adequate circulating blood volume, adequate cardiac
function, and adequate local vasculature
- normovolemic anemia is not associated with impaired healing unless the hematocrit drops
below 15%
- smoking impairs oxygenation by acutely stimulating vasoconstriction
- Radiation
- damages the DNA of exposed cells
- collagen is synthesized to an abnormal degree in irradiated tissue, causing a characteristic
fibrosis
- media of blood vessels thickens and some become occluded, leading to a decreased number of
blood vessels in irradiated tissue
- epidermis becomes thinned
- irradiated skin is dry because of damage to sebaceous and sweat glands
- decreased vascularity and increased fibrosis limits the ability of platelets and inflammatory
cells to gain access to wounds in irradiated tissue
- quantity of cytokines released is diminished and causes impairment of virtually all cellular
aspects of healing
- also, irradiated tissue is predisposed to infection
- vitamin A has been used to reverse the healing impairment caused by radiation therapy
- Systemic Factors
- Malnutrition
- inadequate nutrition is devastating to the healing process
- albumin < 2.0 g/dL represents severe protein malnutrition
- collagen synthesis stops in the absence of protein intake
- arginine supplementation increases collagen deposition
- vitamin C is necessary to produce new, strongly cross-linked collagen
- vitamin A is essential for normal epithelialization, proteoglycan synthesis, and normal
immune function
- vitamin D is required for bone healing
- zinc is an essential cofactor in many enzymes critical to wound healing
- Cancer
- cancer patients have impaired healing
- decreased oral intake may be caused by cachexia or mechanical factors
- protein catabolism may be accelerated
- cancer patients may be relatively anergic: macrophages do not migrate or function normally
- Old Age
- older people take longer to heal than younger people
- increased rates of wound dehiscences and incisional hernias in the elderly
- in elderly patients, wound disruption occurs with less force than in younger patients
- increased incidence of underlying diseases predisposes the elderly to impaired healing
- Diabetes
- risk of infection in clean incisions is 5 times higher in diabetics
- associated with impaired granulocyte chemotaxis and phagocytosis
- large and small-vessel disease causes local hypoxia
- pre-op correction of blood sugar levels improves wound outcomes
- Steroids
- inhibit all aspects of the healing process
- primary problem is a deficiency in inflammatory cell function
- by diminishing the supply of cytokines, steroids and other immunosuppressive agents profoundly
impair macrophage activity and thus subsequent healing capacity
- topical application of vitamin A stimulates collagen synthesis and epithelialization –
this may help overcome the deleterious effects of steroids
- Chemotherapy
- impairs healing primarily through inhibition of cellular proliferation as well as DNA and protein
synthesis within the wound
- if possible, surgery should be delayed for several weeks after the last chemotherapy session
- Obesity
- obese patients have higher incidences of wound complications (30% dehiscence, 17% wound
infections, 30% incisional hernias, 19% seromas)
- obese patients also have a much higher rate of anastomotic leaks
- this is true even when controlled for comorbid conditions such as diabetes and
cardiovascular disease
Wound Complications
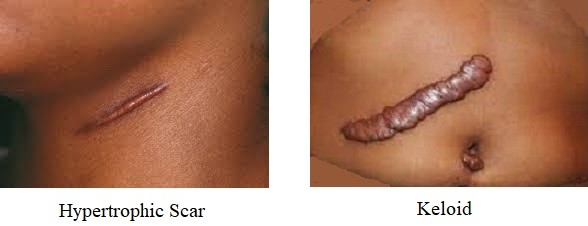
- Hypertrophic Scars and Keloids
- result from excessive healing
- hypertrophic scars rise above the level of the skin, but remain confined to the margins of the
original wound
- keloids extend beyond the confines of the original wound
- both problems result from excess collagen synthesis by wound fibroblasts
- increased TGF-β has been implicated
- hypertrophic scars tend to regress over time; keloids usually do not regress
- keloids tend to recur after excision alone
- intralesional injection of triamcinolone, either alone or in conjunction with surgery, is often
effective treatment
- radiation alone is ineffective, but may be combined with surgical excision
- pressure dressings, topical silastic gel are sometimes used as well
- Wound Dehiscence
- dehiscence is a fascial separation due to abdominal wall tension overcoming tissue resilience,
suture strength, or knot security
- evisceration indicates extrusion of visceral contents through the fascial defect and skin
- local factors contributing to wound disruption include hemorrhage and infection
- systemic factors include malnutrition, hypoproteinemia, morbid obesity, malignancy, uremia,
diabetes, steroids, and increased intra-abdominal pressure (coughing, ascites)
- Technical Factors
- since tension is proportional to incision length, dehiscence is more common when the
incision length exceeds 18 cm
- in the majority of cases, the sutures and knots are intact, but the suture has pulled
through the fascia as a result of fascial necrosis
- fascial necrosis occurs when the sutures are placed too close to the edges or are under too
much tension
- Clinical Presentation
- most dehiscences occur at a mean of 8 days post-op
- profuse serosanguinous drainage from the wound is the classic sign, often preceded by a
popping sensation
- CT or US may be used if the diagnosis is not clear
- Management
- stable patients without evisceration should be returned to the OR for fascial closure
- unstable patients without evisceration may be treated nonoperatively with a sterile wound
dressing and binder
- if the patient eviscerates, moist sterile towels should be placed over the abdominal
contents and the patient returned emergently to the OR
- fascial retention sutures have historically been used to close dehiscences, but they can
cause skin necrosis and pain without significantly reducing the risk of recurrent fascial
disruption or incisional hernia
- Optimal Fascial Closure Technique
- STITCH trial
- simple running closure with a #1 or #2 long-lasting absorbable suture (PDS e.g.)
- mass closure to incorporate all layers of the abdominal wall (except skin)
- tissue bites of 5 mm every 5 mm
- suture length to wound length ratio of 5:1
- non-strangulating tension on the suture
- Peritoneal Adhesions
- result from peritoneal injury from surgery or intra-abdominal infections
- injury elicits an inflammatory response that results in fibrin deposition between damaged serosal
surfaces
- fibrinolytic activity often degrades these filmy adhesions
- if insufficient fibrinolytic activity is present, then permanent fibrous adhesions will form by
collagen deposition within 1 week of injury
- reducing tissue trauma will reduce adhesion formation
- barrier membrane and gels separate and create barriers between damaged mesothelial surfaces
(Seprafilm, Interceed)
- placement of barrier substances directly over anastomoses is contraindicated because of a higher
risk of leak
Wound Management
- Irrigation
- decreases the bacterial load and removes loose material
- low-pressure irrigation (bulb syringe) is adequate for most cases
- high-pressure irrigation (pulse lavage) is indicated for highly contaminated wounds
- Debridement
- wounds that have devitalized tissue, contamination, or residual suture material must be debrided down to healthy,
bleeding tissue
- sharp surgical debridement is the primary technique
- enzymatic debridement with collagenase (Santyl) is a good option for patients who are not surgical candidates
- biologic debridement (maggot therapy) is occasionally used as a bridge between debridement procedures or for
debridement of chronic wounds when surgical debridement cannot be performed
- Topical Agents
- Silver
- used to prevent and treat infected wounds
- very broad spectrum with low toxicity
- bactericidal
- active against yeast, fungi, MRSA, VRE
- silver sulfadiazine (Silvadene) is commonly used on burn wounds
- needs to be complexed to a delivery system – impregnated dressings, foam, cream
- Honey
- has been used since ancient times for wound management
- has broad-spectrum antimicrobial activity due to its high osmolarity and high concentration
of hydrogen peroxide
- Antibacterial Solutions
- acetic acid, Dakin’s solution, betadine, Iodosorb
- partially cytotoxic, and so may impair wound healing
- may be beneficial in selected circumstances (pseudomonas)
- Antibacterial Ointments
- Bacitracin, Neosporin, Polysporin
- soothing to apply, lubricates wound surface, occlusive
- useful for small open wounds or burns
- Growth Factors
- goal is to accelerate healing of chronic wounds by flooding the wound with growth factors
- PDGF (Regranex) is FDA approved for diabetic foot ulcers
- epidermal growth factor and granulocyte-macrophage colony stimulating factor may be
beneficial in chronic venous ulcers
- Wound Dressings
- wound healing is most successful in a warm, moist, clean environment
- wound dressings need to keep the wound moist, but also absorb excess moisture to prevent maceration
of healthy tissue
- wound dressings should also eliminate dead space and prevent bacterial invasion or proliferation
- primary choice is between occlusive and absorptive dressings
- Occlusive Dressings
- good choice for most wounds
- allows for rapid epithelialization, moisture retention, mechanical protection, and a barrier
to bacteria
- hydrocolloids (Duoderm): liquefies to form a moist gel
- alginates: absorb a great deal of fluid, facilitate autolytic debridement
- hydrogels: rehydrating agents for dry wounds
- Absorptive Dressings
- used in wounds with a great deal of exudate or high bacterial counts (venous stasis ulcers)
- prevent maceration of the surrounding skin
- wide mesh gauze (4 x 4) is commonly used, but it loses effectiveness when it gets saturated
- new materials are foam based: can absorb large amounts of fluid, and are nonadherent
- Negative Pressure Wound Devices
- can be used in large soft-tissue injuries, contaminated wounds, fistulas
- remove exudate and infectious materials
- reduces edema
- provides a moist wound environment
- promotes increased capillary blood flow, angiogenesis, and granulation tissue
- reduces hospital stay and costs
- efficacy in accelerated wound healing has been documented in clinical trials
- cons: can be painful, requires a portable pump, fluid loss in large wounds, cost if not covered by
insurance
- Skin Substitutes
- burn wounds are the major indications for these products
- allografts, xenografts, amnion can provide temporary coverage
- bioengineered skin substitutes are available to provide temporary or permanent coverage
- provide biologic elements to the wound
- Alloderm and Integra are acellular and provide dermal matrix elements
- Apligraf provides dermal and epithelial components
- main limitation of these products are their expense and need for multiple applications
- Hyperbaric Oxygen (HBO)
- wound ischemia is the most common cause of wound healing failure
- 100% oxygen is pressurized to 1.5 – 2.5 atmospheres for 60 to 120 minutes over multiple treatments
- tissue oxygen levels can be 10x higher than usual, and can persist for 2 – 4 hours after
treatment ends
- vascular evaluation and possible revascularization is mandatory prior to starting HBO
- transcutaneous oxygen pressure measurements (TCOM) are used to assess who is a candidate for HBO
- TCOM < 35 mm Hg at room air indicates tissue hypoxia
- TCOM > 200 mm Hg in the chamber suggests patient would benefit from HBO
- used in carbon monoxide poisoning, radiation injury, compromised skin grafts and flaps, refractory
osteomyelitis
- there is some evidence that HBO therapy reduces the risk of amputation for patients with chronic
nonhealing diabetic foot ulcers, provided that the limb has undergone revascularization
- middle ear barotrauma is the most common complication of HBO
- pulmonary complications include pneumothorax or tension pneumothorax
- seizures can result from oxygen toxicity
References
- Simmons and Steed, pgs 41 - 55
- O’Leary, 4th ed. Pgs 150 - 169
- Schwartz, 10th ed., Pgs 241 – 268
- Sabiston, 20th ed., Pgs 130 – 160
- UpToDate. Basic Principles of Wound Management. David G. Armstrong, DPM, MD, PhD, Andrew J. Meyr, DPM. June 08, 2021, pgs 1 – 42
- UpToDate. Complications of Abdominal Surgical Incisions. Mizell, Jason. May 2018, pgs 7 – 10

