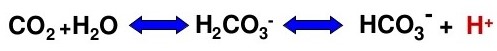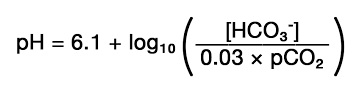Acid-Base Physiology
- Acid-Base Balance
- extracellular pH must be maintained within a narrow range (7.35 – 7.45) or else proteins and enzymes
will not function correctly
- large amounts of acid are produced endogenously as a result of metabolism of fats and carbohydrates
to CO2 and H20
- these acids must be neutralized by several buffer systems and excreted by the lungs and kidneys
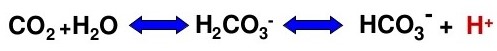
- Henderson-Hasselbalch Equation
- pH is defined by the ratio of bicarbonate to pCO2 concentration and not the
absolute value of either
- accurate diagnosis of a particular acid-base disorder requires the simultaneous measurement
of pH, pCO2, and bicarbonate concentration
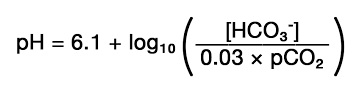
- Daily Acid Load
- daily metabolism produces 15,000 mmol of CO2, which combines with water to
produce H2CO3
- regulation of carbonic acid is achieved by respiratory control of CO2
- daily protein catabolism produces 50 to 100 mEq of sulfuric acids, which reduces the
body’s reserve of bicarbonate
- bicarbonate concentration is controlled by reabsorption and regeneration in the kidney
- Buffers
- acid-base conjugate pairs that can absorb or donate protons
- principal extracellular buffer is bicarbonate, with small contributions made by inorganic
phosphates and proteins
- principal intracellular buffers are proteins, phosphates, and hemoglobin within erythrocytes
- bone is a large buffer reservoir
- Compensation
- changes in hydrogen ion concentration are blunted by renal and pulmonary changes
- these mechanisms will return the pH toward normal but never completely to normal
- if there is no metabolic component, when the pCO2 drops by 10 mm Hg, then the pH rises by
0.08
- renal compensation in respiratory acidosis or alkalosis is a slower response and evolves
over 3 – 5 days
- Base Deficit
- refers to the amount of base required to titrate the pH back to normal (when the contribution of
respiratory factors is taken out of the equation)
- calculated value that represents the absolute deficit or excess of total body HCO3
- the bicarbonate space is ~ 1/3 of body weight (90 kg person has a bicarbonate space of 30 L)
- a base deficit of 6 in this 90 kg person represents a bicarbonate deficit of 180 mEq
- following the base deficit is one way to follow the efficacy of fluid resuscitation (in place of lactate)
Acid-Base Disorders
- Metabolic Acidosis
- ↓ pH, ↓ HCO3-
- results from the excess production of acids (lactic acidosis, diabetic ketoacidosis), reduced acid
excretion (renal failure) or loss of bicarbonate (pancreatic, biliary, or small bowel fistula,
diarrhea)
- initial buffering is accomplished by bicarbonate, although progressively increasing contributions
are made by intracellular buffers and bone
- initial compensation is respiratory, with an increase in the rate and depth of breathing
- increased renal absorption and regeneration of HCO3- is a later compensation
- causes can be divided into two groups by determining the anion gap:
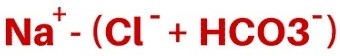
- normal anion gap is 8 to 12 mEq/L
- normal anion gap is made up of unmeasured anions (albumin, phosphates, sulfates)
- Increased Anion Gap Acidosis
- common causes include shock (↑ lactic acid), diabetes (↑ ketoacids), renal failure
(retention of sulfuric and phosphoric acids), and ingestion of methanol, ethylene glycol, or
aspirin
- indicates the loss of HCO3- ions without a concurrent increase
in Cl- ions
- treatment is primarily directed towards correcting the underlying disorder
- hyperventilation and bicarbonate are used to keep the pH > 7.3
- large doses of bicarbonate are associated with hypernatremia,
fluid overload, and hypocalcemia
- Normal Anion Gap Acidosis (Hyperchloremic)
- results primarily from intestinal loss of bicarbonate (pancreatic, small bowel fistulas;
diarrhea)
- less commonly, a renal cause is implicated (renal tubular acidosis, ureteroenterostomy,
spironolactone)
- urine pH is high if the kidney is responsible, and low if the intestine is responsible
- extracellular volume deficit associated with these disorders results in renal conservation
of sodium chloride
- ultimately the chloride concentration rises and the bicarbonate concentration falls
- treatment is to replace the fistula output with fluid with a similar bicarbonate
concentration
- massive resuscitation with NaCl is an iatrogenic cause of nongap acidosis
- Compensatory Respiratory Response to Metabolic Acidosis
- for every 1 mEq/L drop in serum HCO3-, the pCO2 drops by 1.2 mm Hg
- the inability to generate the expected respiratory response usually indicates significant
underlying respiratory or neurologic disease
- Metabolic Alkalosis
- ↑ pH, ↑ HCO3-
- results from the loss of acid or the gain of bicarbonate
- exacerbated by hypokalemia and intravascular volume depletion
- respiratory compensation is minimal (decreased ventilation results in hypoxia)
- causes may be divided into two groups, chloride responsive and chloride resistant
- Chloride Responsive
- urine chloride < 10 to 20 mEq/L
- common causes include vomiting, nasogastric suctioning, gastric outlet obstruction, pyloric
stenosis, diuretics
- associated with volume deficits
- if there is a deficit of chloride, sodium will be reabsorbed in the distal tubule in exchange
for potassium or hydrogen, depending on their relative availability
- this process results in the generation of one bicarbonate ion for each sodium ion that is reabsorbed,
which perpetuates the alkalosis
- treatment consists of volume replacement with an adequate amount of chloride, which allows more sodium
to be reabsorbed with chloride in the proximal tubule, decreasing the generation of bicarbonate in the
distal tubule
- potassium should be added to the fluids once the patient is making urine
- Chloride Unresponsive
- associated with normal to increased volume status
- most causes are secondary to high levels of steroid secretion (Cushing’s disease, exogenous
steroids, primary hyperaldosteronism)
- results from high maximum reabsorption of sodium and bicarbonate and excessive loss of
chloride in the urine
- Compensatory Respiratory Response to Metabolic Alkalosis
- for every 1 mEq/L rise in serum HCO3-, the pCO2 rises by 0.7 mm Hg
- since hypoventilation results in hypoxemia, the pCO2 usually does not rise
above 55 mm Hg
- Respiratory Acidosis
- ↓ pH, ↑ pCO2
- results from decreased minute ventilation, not increased carbon dioxide production
- acute elevations of carbon dioxide are not well handled because renal compensation takes several
days
- numerous conditions may cause inadequate ventilation: airway obstruction, incisional pain, oversedation,
incorrect ventilator settings
- treatment involves restoration of minute ventilation and maintenance of oxygenation
(may require mechanical ventilation)
- it is especially important to avoid respiratory acidosis in head injury patients (hypercapnia causes
cerebral vasodilation, worsening cerebral edema)
- Compensatory Metabolic Response to Respiratory Acidosis
- for every 10 mm Hg rise in pCO2, the serum HCO3- should rise by
1 mEq/L
- Respiratory Alkalosis
- ↑ pH, ↓ pCO2
- causes include hypoxemia, pain, apprehension, CNS injury, incorrect ventilator settings, sepsis
- severe respiratory alkalosis may result in cardiac arrhythmias and cardiac arrest secondary to
hypokalemia (potassium is driven intracellularly in exchange for hydrogen ions) or hypocalcemia
- cerebral ischemia may be worsened due to cerebral vasoconstriction
- attention must be given to the primary disorder causing the hyperventilation
- Compensatory Metabolic Response to Respiratory Alkalosis
- for every 10 mm Hg drop in pCO2, the serum HCO3- should drop by
2 mEq/L
References
- Cameron, 13th ed., pgs 1417 - 1420
- Simmons and Steed, pgs 280 - 282
- UpToDate. Simple and Mixed Acid-Base Disorders. Emmett, Michael and Palmer, Biff. October 10, 2018.
Pgs 1 - 22.