Barrett’s Esophagus
- Pathophysiology
- intestinal columnar epithelium replaces the stratified squamous epithelium of the esophagus
- chronic reflux injures the squamous epithelium and promotes repair through columnar metaplasia
- represents end-stage GERD
- continued reflux of acid (and bile) results in progression to low-grade and then high-grade dysplasia
- the chance of developing esophageal adenocarcinoma is 0.25% per year in patients without dysplasia,
and 4% - 8% per year in patients with high-grade dysplasia
- overall, 10% of patients with GERD develop Barrett’s esophagus
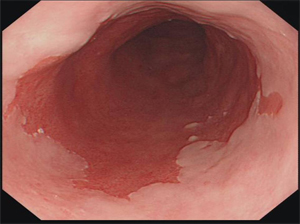
- Diagnosis
- made by endoscopy and pathology
- any length of endoscopically visible columnar epithelium above the GEJ that is confirmed histologically
to be within the esophagus is defined as Barrett’s esophagus
- Treatment of Nondysplastic Barrett’s Esophagus
- Medical Management
- treatment goal is to control GERD effectively
- PPIs are the first-line treatment
- annual surveillance endoscopy is usually recommended, but there is little evidence that it is beneficial
- Antireflux Surgery
- effective treatment for most patients with GERD and BE
- may produce higher rates of BE regression than medical therapy
- however, the long-term effects in preventing esophageal adenocarcinoma are controversial
- Endoscopic Therapies
- radiofrequency ablation and photodynamic therapy can eradicate BE cells and promote reversion back
to squamous epithelium
- no data to suggest that endoscopic ablative therapies reduce cancer risk or are more cost effective
than periodic long-term endoscopic surveillance
- Treatment of Dysplastic Barrett’s Esophagus
- Low-Grade Dysplasia
- medical therapy or antireflux surgery are effective treatment options for GERD
- endoscopic management (ablation or resection) is usually recommended for dysplasia,
but surveillance is another option
- if endoscopic surveillance is chosen, then it should be repeated every 6 – 12 months, with 4-quadrant biopsies
taken at 1 cm intervals
- High-Grade Dysplasia
- Endoscopic Eradication
- obvious mucosal abnormalities should be excised with endoscopic resection
- radiofrequency ablation (RFA) is then used to ablate the remaining metaplastic epithelium
- Esophagectomy
- most patients with high-grade dysplasia are now treated endoscopically
- transhiatal esophagectomy is the most common surgical procedure chosen
- minimally invasive approaches, and vagal-sparing techniques, are becoming more common
- Intramucosal Carcinoma
- endoscopic resection allows for histologic evaluation
- if there is no invasion of the submucosa, then endoscopic therapy is usually the preferred approach
- submucosal invasion will require esophagectomy
Esophageal Carcinoma
- Epidemiology
- Squamous Cell Carcinoma
- accounts for the great majority of cases worldwide
- very high incidence in Northern Iran and certain areas of South Africa, China, and Kazakhstan
- etiologic agents include additives to local foodstuffs (nitroso compounds) and ingestion of hot liquids
- in western countries, smoking and alcohol abuse are strongly linked
- premalignant conditions include achalasia, caustic esophageal burns (lye), and radiation esophagitis
- typically found in the upper and middle thirds of the esophagus
- Adenocarcinoma
- once rare, but is now the fastest growing cancer in the United States
- GERD and obesity are felt to play major etiologic roles
- now accounts for 60% - 70% of esophageal cancers in the U.S.
- originates in metaplastic columnar-lined epithelium (Barrett’s Esophagus) which occurs in 10% of patients
with GERD
- Barrett’s esophagus is associated with a 30 - 40X increased risk of esophageal adenocarcinoma
- Clinical Manifestations
- early-stage tumors may be asymptomatic and are usually found on routine endoscopic surveillance for
Barrett’s esophagus
- dysphagia and weight loss are the most common symptoms, and indicate advanced disease
- odynophagia may also be present
- extension of the tumor into the tracheobronchial tree can result in a tracheoesophageal fistula,
with symptoms of choking, coughing, and aspiration pneumonia
- paralysis of a recurrent laryngeal may occur as a result of local invasion
- Diagnosis and Staging
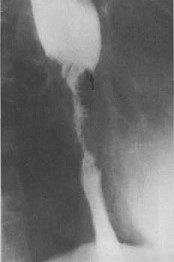
- Esophagram
- recommended for any patient presenting with dysphagia
- provides an overview of anatomy and function
- can distinguish between extrinsic and intrinsic compression
- classic finding of cancer is an apple core lesion
- Endoscopy
- allows biopsy of tumors
- can determine the distance of the tumor from the incisors and GEJ
- can assess for gastric cardia involvement and the suitability of the stomach as a replacement conduit
- CT Scan
- useful to evaluate the extent of the tumor and its relationships with surrounding structures,
regional nodal status, and metastatic disease to the liver and lungs
- only 57% accurate for T staging, 74% for N staging, and 83% for M staging
- PET-CT Scan
- probably the single best systemic staging tool
- overall accuracy for staging regional nodes and presence of distant disease is higher than CT scanning
- Endoscopic Ultrasound (EUS)
- can determine the depth and length of the tumor, status of regional nodes
- EUS-FNA provides the most accurate nodal staging information
- overall T-stage accuracy is 85%, but is more accurate for increasing T stage
- Endoscopic Mucosal Resection
- for early-stage tumors, allows for accurate histologic distinction between mucosal (T1a) and
submucosal (T1b) tumors
- since esophageal lymphatics are in the submucosa, T1a tumors can be treated endoscopically,
while T1b tumors require surgical resection
- Other Staging Tools
- bronchoscopy is used to rule out tracheal invasion or tracheoesophageal fistula in symptomatic patients
- mediastinoscopy may be used to biopsy suspicious nodes not amenable to EUS-FNA
- laparoscopy or thoracoscopy may be used to evaluate possible metastatic lesions in selected patients
- Cancer-Related Biomarkers
- beginning to find a role in esophageal cancer
- HER2 overexpression correlates with cancer progression and may be amenable to treatment with trastuzumab
- Staging
- precise staging allows for the most appropriate treatment
- tumor depth (T stage) directly relates to lymph node involvement
- T1a and superficial T1b tumors rarely metastasize to regional nodes
- deep T1b tumors (>50% of the submucosa invaded) are metastatic to regional nodes ~ 40% of the time
- T2 tumors involve the muscularis propria
- T3 tumors involve the adventitia
- T4 tumors locally invade other mediastinal structures
- Anatomy
- Arterial Supply
- segmental
- cervical esophagus receives its blood supply from the superior and inferior thyroid arteries
- thoracic esophagus receives blood from 4 to 6 esophageal aortic arteries, as well as from
branches of the bronchial and intercostal arteries
- abdominal esophagus receives blood from the left gastric artery and inferior phrenic arteries
- Venous Drainage
- is first into the submucosal venous plexus and then into the inferior thyroid vein (cervical esophagus);
bronchial, azygos, and hemiazygos veins (thoracic esophagus); and the coronary vein (abdominal esophagus)
- Lymphatic Drainage
- esophagus has an extensive lymphatic drainage
- lymphatics are located primarily in the submucosa
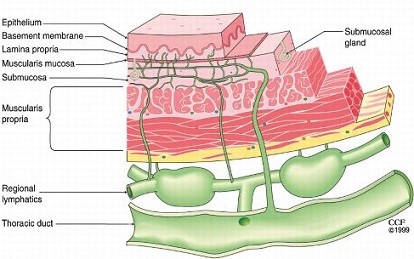
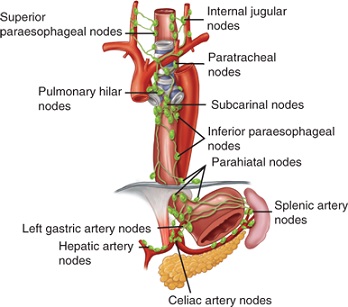
- lymph flow is in the longitudinal direction, which facilitates spread along the esophageal wall
- lymph flow in the upper 2/3 of the esophagus is upwards (internal jugular nodes, paratracheal and
mediastinal nodes)
- lymph flow in the lower 1/3 of the esophagus is downwards (inferior paraesophageal nodes, left gastric nodes,
celiac artery nodes)
- Treatment
- Management Considerations
- Histology
- squamous cell cancer (SCC) is more responsive to chemoradiation than adenocarcinoma
- SCCs are more likely to have a complete clinical response to neoadjuvant chemoradiation,
making the need for surgical intervention uncertain
- Tumor Depth
- tumors confined to the mucosa (T1a) can be treated with endoscopic mucosal resection
- T2, T3 tumors should probably receive neoadjuvant chemoradiation
- Regional Nodal Status
- patients with known nodal disease, or those at high risk because of tumor depth, should probably
receive neoadjuvant chemoradiation
- Systemic Disease
- require definitive chemoradiation
- if totally obstructed, can consider a palliative resection if life expectancy is reasonable
- Patient Condition
- age, comorbidities, nutritional status affect the ability of patients to tolerate treatment
- most patients need preoperative pulmonary function and cardiac stress testing
- greater than a 10% weight loss or albumen level < 3.4 are associated with increased surgical complications
- nutritional status can be improved with an esophageal stent or feeding jejunostomy tube
- Neoadjuvant Treatment
- should be considered for all tumors at high risk for nodal metastasis (deeper than deep T1b)
- near consensus that surgery alone is inadequate for regionally advanced cancers
- concurrent chemoradiation is better than sequential chemotherapy and radiation
- one unanswered question is whether to proceed with an esophagectomy in a patient who has had a complete
clinical response to chemoradiation
- Surgical Resection
- Transhiatal Esophagectomy (THE)
- performed through an upper midline abdominal incision and a cervical incision, avoiding
a thoracotomy
- entire thoracic esophagus is resected
- stomach is used as the replacement organ in most cases
- the anastomosis is placed in the neck, avoiding the complications of an intrathoracic leak and
gastroesophageal reflux
- a pyloromyotomy and feeding jejunostomy are performed routinely
- this operation has been criticized as violating oncologic surgical principles by omitting a
formal lymph node dissection in potentially curable patients
- a laparoscopic procedure is now being done in some centers, and may be associated with lower complication
rates and shorter hospital stays
- Indications and Contraindications
- excellent palliative procedure
- many surgeons use this operation for ‘curative’ resections
- contraindications include tracheobronchial invasion and fixation to surrounding structures
- lowest mortality and major morbidity rates of all the esophageal resections
- Preoperative Considerations
- if the stomach is not available as an esophageal replacement because of previous gastric surgery,
then a barium enema of the colon is necessary to assess the suitability of the colon as the replacement organ
- Operative Considerations
- must preserve the right gastroepiploic vessels
- preserve the spleen
- pyloromyotomy and feeding jejunostomy are routine
- in the neck, the recurrent laryngeal nerves must be protected
- must assess the mobility of the tumor before proceeding with the thoracic phase of the
procedure
- much of the ‘blunt’, ‘blind’ thoracic phase of the procedure may be done sharply and
under direct vision by inserting long, narrow retractors through the esophageal hiatus
- mediastinal dissection must be kept close to the esophageal wall
- as the stomach is brought up into the neck, care must be taken not to twist the stomach in
the posterior mediastinum
- Complications
- intraoperative complications include pneumothorax, hemorrhage, and a tracheal tear
- postoperative complications include hoarseness, anastomotic leak or stricture, chylothorax,
and pleural effusion
- En Bloc Esophagogastrectomy
- requires 3 incisions: right posterolateral thoracotomy, upper midline abdominal incision,
left cervical incision
- gastrointestinal continuity is usually restored with the stomach
- radical thoracic and abdominal lymphadenectomies are performed
- some studies claim significantly better survival rates for early cancers than with transhiatal
esophagectomy
- has the highest mortality and morbidity rate of all the esophageal resections
- entire esophagus is resected, thereby eradicating submucosal spread
- avoids an intrathoracic anastomosis
- Left Thoracoabdominal Approach
- used for lesions in the distal esophagus and cardia
- distal esophagus, proximal stomach, and adjacent lymph node basins are resected
- a pyloroplasty or pyloromyotomy is required for gastric drainage
- an intrathoracic anastomotic leak is the most feared and lethal complication
- intrathoracic anastomoses are invariably associated with reflux
- obtaining negative margins is difficult since esophageal carcinoma spreads extensively in the
submucosal plane
- there are increased respiratory complications from a combined thoracic and abdominal operation
- Ivor-Lewis Esophagogastrectomy
- combines right thoracotomy and abdominal incisions
- used primarily for lesions in the middle third of the esophagus
- has all the drawbacks of a transthoracic esophagectomy
- leak rates are low, but since they occur in the chest, they can be difficult to control
- often done now with a minimally invasive approach (thoracoscopic/laparoscopic)
- Vagal-Sparing Esophagectomy
- similar to THE
- vagal nerves are preserved by stripping the esophagus away from the nerves
- highly selective vagotomy is performed, so a pyloroplasty is not necessary
- advocated for use in intramucosal tumors
- Palliative Therapy
- ~50% of patients with esophageal carcinoma have local tumor invasion or distant metastases,
precluding cure
- primary goal is relief of dysphagia by reestablishing an esophageal lumen
- combination chemotherapy and radiation does not improve survival but can improve local control
- photodynamic therapy, endoscopic laser therapy, and esophageal stenting can all play roles in
palliating dysphagia
Benign Esophageal Tumors
- Leiomyomas
- now classified as GIST tumors (c-KIT-positive); true leiomyomas are rare
- dysphagia and pain are the most common symptoms
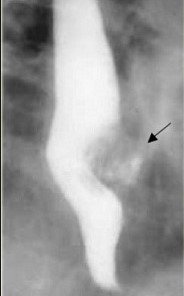
- have a distinctive appearance on barium swallow: smooth, concave defect with intact mucosa and sharp borders
- esophagoscopy should be done to rule out carcinoma, but if a leiomyoma is suspected it should not be biopsied
(scarring at the biopsy site complicates the extramucosal resection of the mass)
- asymptomatic incidentally found small tumors may be safely followed with periodic barium swallows
- symptomatic or large tumors should be removed by enucleation
References
- Sabiston, 19th ed., pgs 1047 – 1065
- Cameron, 11th ed., pgs 47 – 54, 54 – 58
- UpToDate. Barrett’s Esophagus: Surveillance and Management. Spechler MD, Stuart. Oct 15, 2018. Pgs 1 – 25
- UpToDate. Clinical Manifestations, diagnosis, and Staging of Esophageal Cancers. Saltzman MD, John, Gibson MD, Michael. Oct 22, 2018. Pgs 1 – 38
- UpToDate. Surgical Management of Resectable Esophageal and Esophagogastric Junction Cancers. Swanson MD, Scott. Feb 07, 2019. Pgs 1 – 51




