Liver Anatomy and Physiology
Anatomy
- Gross Anatomy
- General Description
- largest gland in the body, accounting for 2% of the body weight of an adult and 5% of the
body weight of a newborn
- its large size reflects the complexity of its functions
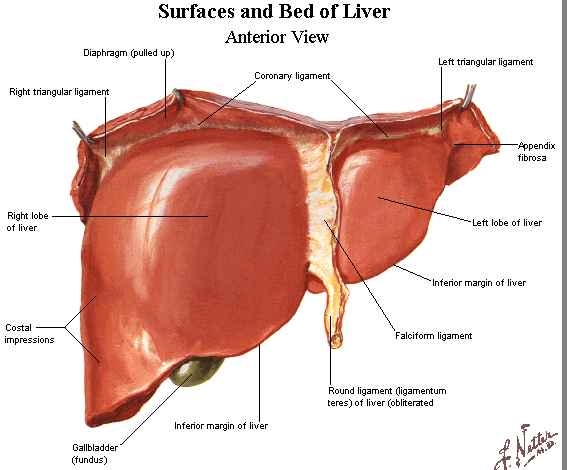
- Surface Anatomy
- completely covered by peritoneum (Glisson’s capsule) except for the bare area on its
posterosuperior surface adjacent to the inferior vena cava and hepatic veins
- Falciform Ligament
- attaches the liver to the anterior abdominal wall from the diaphragm to
the umbilicus
- ligamentum teres, or round ligament, (obliterated left umbilical vein) is enclosed in its lower border
- Coronary Ligaments
- consists of anterior and posterior leaves which, in continuity with the falciform
ligament, connect the liver to the diaphragm
- anterior and posterior leaves fuse laterally to form the right and left
triangular ligaments
- bare area is the area encompassed by the falciform, coronary, and triangular
ligaments
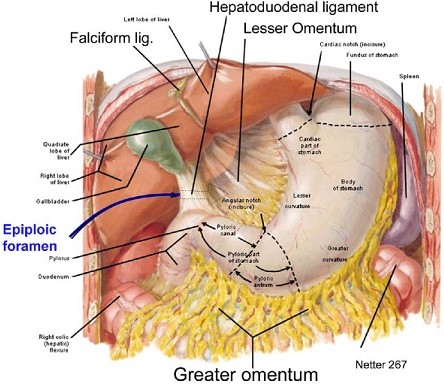
- Lesser Omentum
- comprised of the gastrohepatic ligament and the hepatoduodenal ligament
- hepatoduodenal ligament contains the portal triad: hepatic artery, common bile duct,
and portal vein
- gastrohepatic ligament may contain aberrant or accessory vessels to the liver
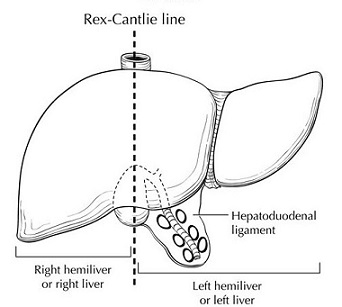
- Lobar Anatomy
- division of the liver into right and left lobes is based on the right and left branches of
the hepatic artery and portal vein
- this division has no topographical landmark, but instead occurs along Cantlie’s line,
which is a plane passing from the gallbladder fossa anteroinferiorly to the vena cava posteriorly
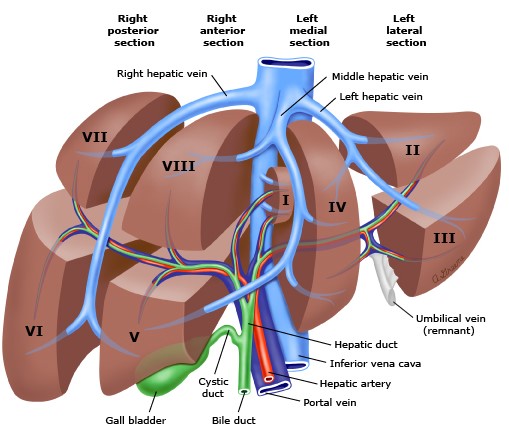
- Couinaud’s Segmental Classification
- functional division of the liver based on the hepatic venous drainage
- liver is divided into two lobes by the middle hepatic vein
- right hepatic lobe is divided into two sectors by the right hepatic vein;
the left hepatic lobe is divided into two sectors by the left hepatic vein
- each sector is divided into two segments, which correspond to the portal vein and
hepatic artery branches
- liver contains a total of 8 segments: segment I = caudate lobe,
segments II to IV = left lobe, and segments V to VIII = right lobe
- each segment has its own vascular inflow, outflow, and biliary drainage
- each segment is a self-contained unit that can be resected without
damaging the others
- resection lines parallel the hepatic veins
- Blood Supply
- Hepatic Artery
- provides 25% of hepatic blood flow and 50% of the liver’s oxygen
- originates from the celiac axis
- gives off the gastroduodenal and right gastric arteries
- ascends in the hepatoduodenal ligament to the left of the common bile duct and
anterior to the portal vein
- bifurcates outside the liver into right and left branches
- inside the liver, the hepatic arteries run parallel to the portal vein branches
- ligation of the hepatic artery proximal to the gastroduodenal artery will not damage
the liver because of abundant collaterals
- most patients can also tolerate ligation of either the right or left hepatic artery
without serious sequelae
- ‘normal’ anatomy only occurs ~60% of the time
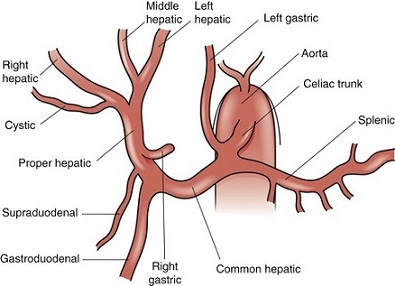
- Replaced Right Hepatic Artery
- originates from the SMA in 11% to 22% of patients
- usually lies to the right and posterolateral to the bile duct and portal vein
- can be palpated along the posterior aspect of the hepatoduodenal ligament by placing
a finger through the foramen of Winslow
- occurs as an accessory vessel in 7% of cases
- Replaced Left Hepatic Artery
- occurs in 4% to 10% of patients
- originates from the left gastric artery
- runs through the gastrohepatic ligament
- occurs as an accessory vessel in 8% of cases
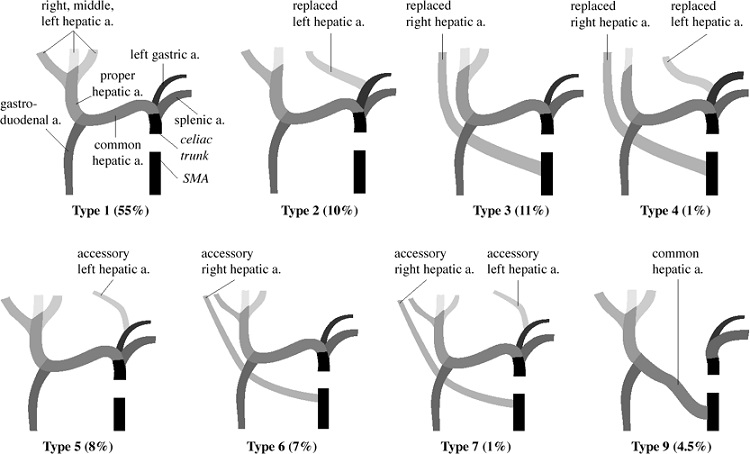
- Portal Vein
- accounts for 75% of hepatic blood flow and 50% of the liver’s oxygen requirement
- drains the gastrointestinal tract, pancreas, and spleen
- formed behind the neck of the pancreas by the confluence of the superior mesenteric
and splenic veins
- lies posterior to the common bile duct and hepatic artery in the hepatoduodenal
ligament
- blood from the superior mesenteric vein flows preferentially into the right lobe
of the liver
- contains numerous rudimentary collaterals with the systemic circulation that can
become clinically significant if portal hypertension develops:
- submucosal veins of the proximal stomach and distal esophagus
- umbilical and periumbilical veins
- middle and inferior hemorrhoidal veins
- retroperitoneal veins
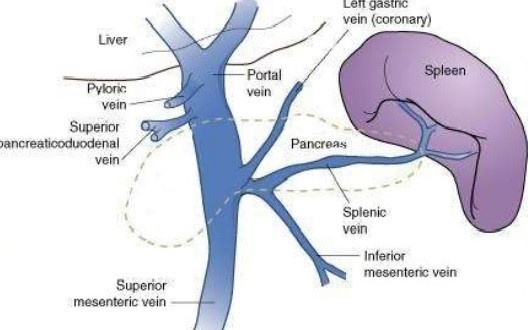
- Hepatic Veins
- begin in the liver as the central veins into which the sinusoids empty
- right hepatic vein (RHV) provides the principal drainage of the right lobe
- in addition, multiple small veins drain directly from the right lobe into the vena cava
- middle hepatic vein (MHV) drains the medial segment of the left lobe and a portion of the
anterior segment of the right lobe
- left hepatic vein (LHV) drains the left lateral segment
- caudate lobe drains directly into the vena cava
- the most common pattern for the 3 hepatic veins is an independent RHV and a common
trunk for the MHV and LHV
- Microscopic Anatomy
- Hepatic Lobule
- functional unit of the liver
- made up of a central vein surrounded by 4 to 6 portal triads that form a polygonal unit
- blood flows from the terminal hepatic arterioles and portal venules into the sinusoids
- blood in the sinusoids bathes the hepatocytes before it empties into the hepatic veins
- bile is made in the hepatocytes and empties into terminal canaliculi
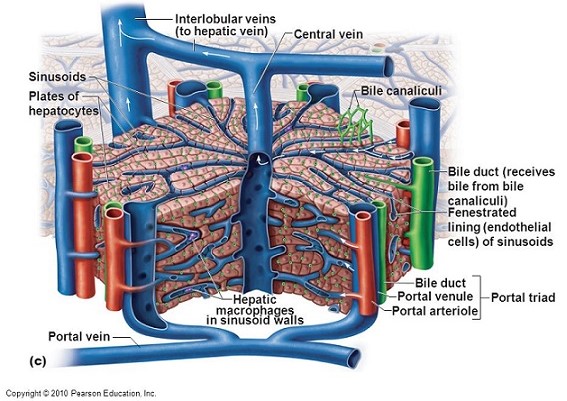
- Major Cell Types
- Hepatocytes
- perform the major metabolic and excretory functions of the liver
- arranged in cords or plates one cell thick
- surrounded by the sinusoids
- Endothelial Cells
- make up the specialized capillary bed of the hepatic sinusoids
- do not form a continuous cell layer – the gaps between the cells are wide
- the sinusoids are porous and allow migration of most large molecules into the
space of Disse between the sinusoids and the hepatocytes
- pressure within the sinusoids is only 2 to 3 mm Hg
- flow into the space of Disse is very sensitive to increases in hydrostatic pressure
as seen in cirrhosis or the Budd-Chiari syndrome
- fluid in the space of Disse is removed by hepatic lymphatics
- Kupffer Cells
- lie within the sinusoids
- tissue macrophages that function as phagocytic cells and antigen-presenting cells
- function as part of the reticuloendothelial system
- Bile Canaliculus
- forms the origin of the biliary system
- formed by the plasma membranes of adjoining hepatocytes
- separated from the space of Disse by tight junctions and desmosomes
- drain into ductules within the portal triads and then follow the portal vein and
hepatic artery retrograde to the hilum of the liver
Physiology
- Metabolism
- Carbohydrates
- liver plays a central role in glucose homeostasis
- provides a continuous source of glucose for the CNS and RBCs
- Fed State
- liver removes 50% of the glucose presented to it by the portal vein
- glucose absorbed by the hepatocytes is converted to glycogen for storage
(up to 65 grams of glycogen / kg of liver tissue)
- excess glucose is converted into fatty acids and stored in adipose tissue
- Fasting State
- in early fasting (<24 hr), liver glycogen is the primary source of glucose
- after <24 hours of fasting, liver glycogen is used up and muscle protein
(mainly alanine) must be converted by the liver into glucose (gluconeogenesis)
- lactate, produced by anaerobic metabolism or in red cells, is converted into
glucose in the liver by the Cori cycle
- Lipids
- free fatty acids are esterified with glycerol to form triglyceride, which is then exported
with very low density lipoprotein (VLDL)
- liver is the major organ involved in the synthesis, esterification, and excretion
of cholesterol
- site of phospholipid production
- site of beta oxidation of fatty acids to produce ketone bodies, which provide energy
in the fasting state
- Proteins
- in prolonged fasting, muscle proteins, particularly alanine, are converted in the
liver to glucose
- site of synthesis of many important serum proteins, including albumin,
coagulation factors, lipoproteins
- acute-phase reactants are produced by the liver under the influence of IL-6 and
systemic inflammatory states
- principal site of urea synthesis
- Vitamins
- liver is responsible for the modification of most vitamins into their active co-enzyme form
- liver is also responsible for the storage and excretion of vitamins and their metabolites
- absorption of the fat-soluble vitamins A, D, E, and K is dependent upon bile production
- initial step in vitamin D activation (25-hydroxylation) occurs in the liver
- Bile Production
- bile serves 2 main functions: 1) route of excretion for certain substances
(bilirubin, cholesterol, steroids, antibiotics); 2) facilitates intestinal absorption of lipids
and fat-soluble vitamins
- normal daily production of bile is 1500 ml
- formed at 2 sites: bile canaliculi and bile ductules
- principal organic compounds include bile acids, cholesterol, phospholipids, and proteins
- under the influence of secretin
- Coagulation
- all the procoagulant factors except von Willebrand’s factor are produced by the liver
- factors II, VII, IX, and X are dependent upon vitamin K for their activation
- factor VII has the shortest half-life (5 to 7 hrs)
- synthetic function of the liver may be assessed by measuring the prothrombin time,
which is dependent upon factor VII
- Detoxification
- drug and toxin metabolism is primarily a hepatic function
- detoxification reactions include oxidation, reduction, hydrolysis, and conjugation
with an endogenous molecule
- cytochrome P-450 enzyme system is responsible for most of the oxidation reactions
- Immune Function
- liver contains the largest number of fixed macrophages in the body
- Kupffer cells lie entirely within the sinusoids and thus are in constant contact with the bloodstream
- Kupffer cells also act as modulators of hepatocyte function by means of cytokine secretion in
response to septic stimuli
Assessment of Liver Function
- Routine Screening Tests
- ALT and AST are markers of hepatocellular necrosis
- elevated alkaline phosphatase level suggests cholestasis or biliary obstruction
- elevated conjugated (direct) bilirubin suggests mechanical obstruction or cholestasis;
elevated unconjugated (indirect) bilirubin suggests hemolysis or drug effects
- hypoalbuminemia is a marker of severe liver disease or chronic malnutrition
- elevated prothrombin time or INR is a marker of advanced liver disease
- LFTs are non-specific and have little or no prognostic value
- Scoring Systems
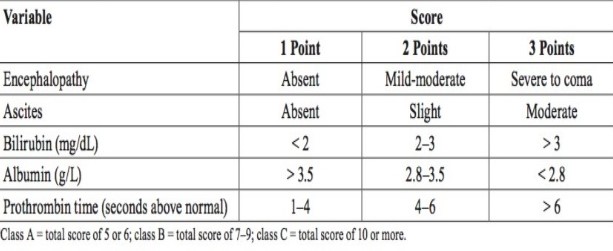
- Child-Pugh Score
- originally designed to predict mortality in patients with cirrhosis and to select patients who might
benefit from an elective portocaval shunt
- also predicts mortality after major abdominal operations
- based on clinical observations and standard liver function tests
- Child class A patients can generally tolerate a major hepatectomy or a major abdominal operation with
low mortality (~10%)
- elective surgery is contraindicated in Child class C patients because of a prohibitively
high mortality (70% - 80%)
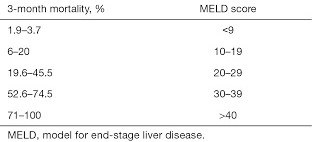
- MELD Score
- developed to predict 3 month survival in patients with cirrhosis
- major use is to prioritize patients for liver transplant
- can also be used to access risk for elective surgery
- calculated using serum bilirubin, serum creatine, and INR
References
- Sabiston, 20th ed., pgs 1418 - 1436
- Schwartz, 10th ed., pgs 1263 - 1277
- UpToDate. Hepatic Resection Techniques. Steven A. Curley MD, FACS, Evan S. Glazer, MD, PhD, MPH, FACS.
Dec 02, 2019. Pgs 1 – 44
- Simmons and Steed, pgs 246 – 254










