Liver Resection and Ablation
Liver Resections
- Anatomic Considerations
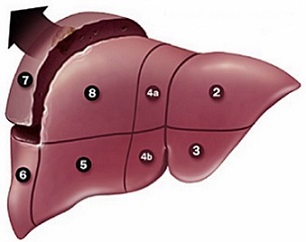
- Segmental Anatomy
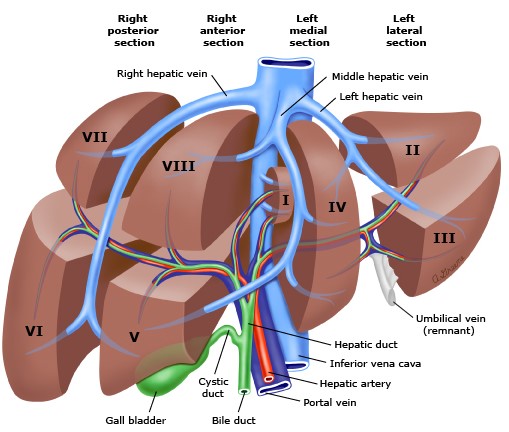
- hepatic veins divide the liver into 4 sectors
- middle hepatic vein divides the liver into a right and left hemi-liver (lobe)
- each sector is divided into 2 segments by the portal triad structures
- Arterial Supply
- many different variations that, if unrecognized, can lead to excessive bleeding
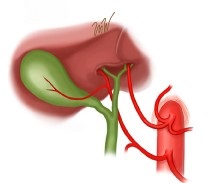
- Replaced Right Hepatic Artery
- may be the main vessel or an accessory vessel
- originates from the SMA
- can be palpated in the hepatoduodenal ligament as a posterolateral structure
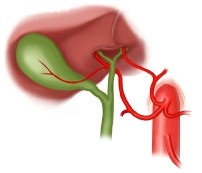
- Replaced Left Hepatic Artery
- originates from the left gastric artery
- identified as a prominent vessel in the gastrohepatic ligament
- may also be the main vessel or an accessory vessel
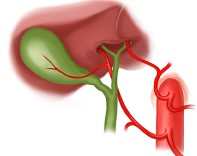
- Replaced Right and Left Hepatic Arteries
- occurs in ~ 1% of patients
- Staging Laparoscopy and Ultrasound
- preoperative laparoscopy may detect peritoneal implants that would preclude hepatic resection
- intraoperative ultrasound may identify additional lesions in the liver that would preclude a curative resection
- General Principles
- Incision
- multiple options are possible depending upon the patient’s body habitus and the site of the lesion
- a right subcostal or bilateral subcostal incision are the most common choices and a superior midline
extension can be added for additional exposure
- for large tumors in segments VII or VIII, the incision can be extended into the right chest for additional exposure
- if a synchronous colectomy is to be performed, then a long midline incision may be adequate
- Mobilization
- the coronary, triangular, and falciform ligaments that attach the liver lobe to the diaphragm and
abdominal wall need to be divided to facilitate operative exposure
- some surgeons reapproximate the falciform ligament to prevent postoperative torsion of the liver remnant
- Cholecystectomy
- facilitates an easier and safer dissection in the porta hepatis
- also eliminates the gallbladder as a source of postop complications
- if necessary, cholangiograms can be performed through the transected cystic duct
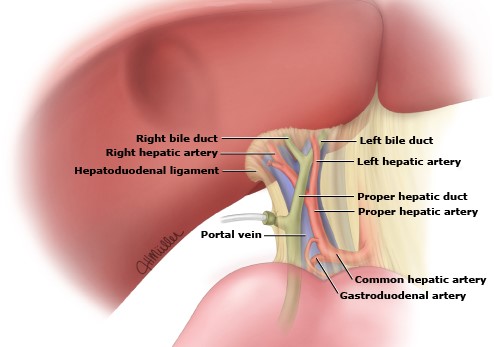
- Porta Hepatis Dissection
- required for all lobectomies and extended lobectomies
- before proceeding, identify any aberrant accessory vessels in the hepatoduodenal ligament
- goal is to get proximal and distal control of the portal vein, proper hepatic artery, and the
common hepatic duct before dissecting the lobar branches
- each lobar branch structure should be individually ligated and divided
- Pringle Maneuver
- occludes the portal structures by clamping the hepatoduodenal ligament
- valuable temporary maneuver in resections that don’t involve ligating the lobar vessels
(segmentectomies, nonanatomic resections)
- short duration occlusions (< 15 min) with a 10 minute rest period between occlusions result in less
hepatic injury than continuous occlusion
- Dissection of the Liver Parenchyma
- Marking the Dissection Plane
- if the lobar vessels have been ligated, then the line of resection will be clearly demarcated
- for segmentectomies, the resection plane will need to be identified with intraop ultrasound and
marked on the liver surface with cautery
- Tissue Division and Hemostasis
- the initial 1 cm of incision depth can be done with the electrocautery, and deep chromic vertical
mattress sutures can be placed to help control bleeding
- the subsequent tissue division can be done by finger fracture or the clamp-crush technique
- a variety of electrosurgical devices can also be used to divide the liver: CUSA, hydrojet,
ultrasonic shears
- as the liver tissue is broken apart, the bile ducts and vessels are identified and controlled
with clips, staples, or sutures
- after the specimen has been removed, liver edge bleeding can be controlled with suture ligation,
topical agents, or argon bean coagulation
- Drains
- no evidence that they need to be routinely placed
- primary purpose is to control a bile leak
- Specific Resections
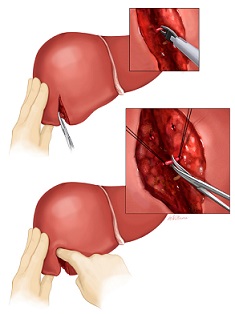
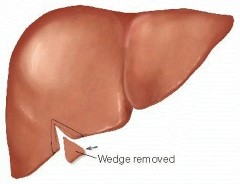
- Wedge Resection
- most often used for benign or small metastatic lesions location on the edge of the liver
- a V-shaped incision is marked and liver sutures are placed for hemostasis
- the parenchyma is divided with the electrocautery
- lesions located away from the edges will require a circular incision
- Segmentectomy
- main use is as a parenchymal-sparing technique for a lesion located in the center of a segment
- transection line is marked on the liver surface using ultrasound
- porta hepatis vessels are controlled but not divided
- liver parenchyma is divided until the segmental vessels are reached
- short Pringle maneuvers may help control bleeding
- segmental vessels are temporarily clamped to ensure that the blood supply to the remaining segments of the
lobe is adequate
- cholangiography is done to ensure that the remaining lobar segments have adequate biliary drainage
- if there is adequate blood supply and biliary drainage to the remaining lobe, the segmental vessels are
divided with sutures or staples
- continue dividing the parenchyma until the segmental hepatic vein is identified and then divided
- Right Hepatic Lobectomy
- divide the triangular and coronary ligaments to fully mobilize the lobe
- ligate the right portal vein and right hepatic artery, which demarcates the right lobe from the left
- the right hepatic duct is divided inside the liver to preserve aberrant branches
- the vessels from the liver to the IVC must be divided, often with a vascular stapler
- ligate and divide the right hepatic vein outside the liver
- using ultrasound as a guide, the parenchymal transection must avoid the middle hepatic vein
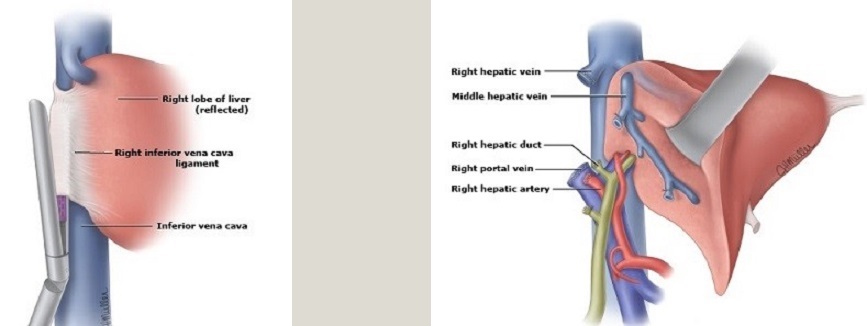
- Left Hepatic Lobectomy
- ligate the left portal vein and left hepatic artery
- parenchymal transection needs to stay to the left of the middle hepatic vein
- left hepatic duct is divided inside the liver
- left hepatic vein is divided inside the liver near its junction with the middle hepatic vein
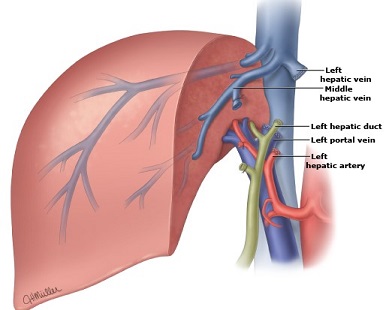
- Extended Right Hepatic Lobectomy
- most common indication is for a right hepatic lesion that extends into the medial segment of the
left lobe (segment IV)
- future liver remnant must be sufficient to avoid post op liver failure (20% in a noncirrhotic;
30% in a patient previously treated with chemotherapy)
- vascular structures to be divided include the right portal vein, right hepatic artery, right and
middle hepatic veins, and the blood supply to the left medial sector (IV)
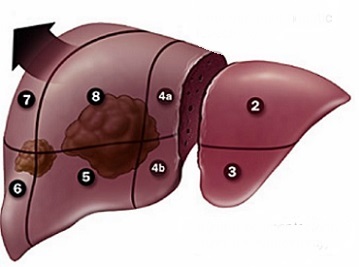
- Extended Left Hepatic Lobectomy
- vascular structures to be divided include the left portal vein, left hepatic artery, left
and middle hepatic veins, anterior branch of the right portal vein and hepatic artery
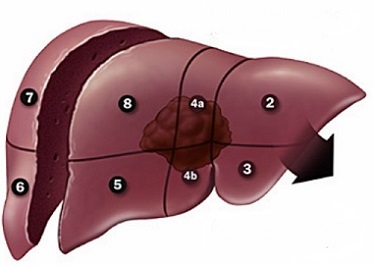
Thermal Ablation and Percutaneous Ethanol Injection
- Indications
- disease must be localized to the liver
- Child’s score A or B
- patient is not a candidate for surgical resection or liver transplantation
- may be used as bridging therapy while awaiting transplant
- appropriate for both HCC and metastatic lesions
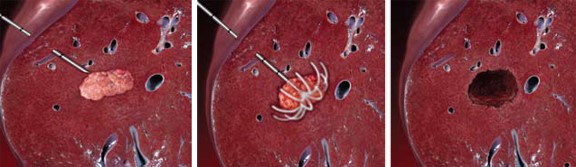
- Radiofrequency Ablation (RFA)
- kills tumor cells by heating them to > 60°C
- long-term local control and disease-free survival are possible in patients with lesions < 5 cm
- tumors > 3 cm will require more than one deployment of the needle
- only one lesion can be treated at a time
- most commonly performed in the interventional radiology suite
- Contraindications
- tumors located near a major blood vessel (heat sink) or hepatic hilum (bile duct injury)
- tumors adjacent to the diaphragm, gallbladder, stomach, duodenum, or colon should probably
be managed in the OR, where these structures can be protected from heat damage
- Assessment of Results
- a complete radiologic response is defined as a well-demarcated ablation zone with a lack of
contrast enhancement
- residual, or recurrent, tumor appears as an irregular, peripherally enhancing nodule on arterial
phase CT or MRI
- Complications
- lethal complications are rare (< 1%): liver failure, portal vein thrombosis, colon perforation
- major complications include liver abscess, pneumothorax, pleural effusion, subcapsular hematoma
- postablation syndrome: fever, chills, nausea, malaise, RUQ pain, elevated transaminase levels
- Microwave Ablation (MWA)
- not routinely available in the U.S., but there is extensive experience in Asia
- one advantage of MWA over RFA is that multiple lesions can be treated simultaneously
- a second advantage is that MWA is much quicker than RFA
- overall survival and recurrence-free survival appear similar for MWA and RFA
- Cryoablation
- has largely been replaced by RFA
- associated with significant morbidity and mortality
- liver surface fracture can lead to exsanguinating hemorrhage and coagulopathy
- myoglobinuria and acute renal failure are also potentially lethal complications
- Percutaneous Ethanol Injection
- has largely been replaced by RFA, if RFA is available
- Indications
- most common use is for small HCCs in locations not suitable for percutaneous RFA
- Disadvantages
- high recurrence rates
- painful
- requires multiple treatments
Transarterial Embolization
- Indications
- majority of the blood supply to an HCC or metastatic lesion comes from the hepatic artery
- to be a candidate for embolization, the main portal vein and its lobar branches must be patent
- embolization is sometimes used alone for unresectable HCCs that are too large or multifocal for RFA,
but it is often combined with RFA
- also indicated as bridging therapy in patients awaiting liver transplantation
- may be combined with contralateral portal vein embolization to facilitate hypertrophy of the future liver remnant
in a patient with a large but resectable tumor
- Particle Embolization
- induces tumor ischemia by occluding the tumor’s blood supply
- has not been shown to increase survival
- Chemoembolization (TACE)
- particle embolization combined with injection of chemotherapy agents, most often using drug-eluting beads
- drug-eluting beads slowly release chemotherapy, potentially reducing chemotherapy-related toxicity
- clinical trials have shown conflicting results as to whether TACE improves outcomes
- Radioembolization
- intraarterial injection of radioactive microspheres induces extensive tumor necrosis
- most common indication is when there is portal vein thrombosis, which prevents the use of TACE
References
- Schwartz, 10th ed., Pgs 1296 – 1302
- UpToDate. Overview of Hepatic Resection. Steven A. Curley, MD, FACS, Evan S. Glazer, MD, PhD, MPH, FACS. Sep 03, 2019. Pgs 1 – 40
- UpToDate. Hepatic Resection Techniques. Steven A. Curley, MD, FACS, Evan S. Glazer, MD, PhD, MPH, FACS. Dec 02, 2019. Pgs 1 – 44
- UpToDate. Nonsurgical Therapies for Localized Hepatocellular Carcinoma: Radiofrequency Ablation, laser and Microwave
Thermal Ablation, Percutaneous Injection Therapies, Cryoablation, High-Intensity focused Ultrasound,
and Irreversible Electroporation. Steven A. Curley, MD, FACS, Keith E. Stuart, MD, Jonathan M. Schwartz, MD,
Robert L Carithers. Mar 26, 2020. Pgs 1 – 29.
- UpToDate. Nonsurgical Therapies for Localized Hepatocellular Carcinoma: Transarterial Embolization, Radiation Therapy,
and Radioembolization. Steven A. Curley, MD, FACS, Keith E. Stuart, MD, Jonathan M. Schwartz, MD, Robert L Carithers.
Apr 14, 2020. Pgs 1 – 42.












