GIST Tumors
- Epidemiology
- most common sarcomatous tumor of the GI tract
- the stomach accounts for 40% - 60% of cases; jejunum/ileum, 25% - 30%; duodenum,
5%; and colon/rectum, 5% - 15%
- approximately 5000 – 6000 cases/year
- mean age at diagnosis is 60 years
- most cases arise sporadically, although familial syndromes exist (neurofibrosis 1,
von Hippel-Landau disease, GIST-paraganglioma syndrome (Carney triad))
- Pathogenesis
- GIST tumors arise from the interstitial cells of Cajal, a gastrointestinal pacemaker cell
- most GIST tumors (80%) over express the kit proto-oncogene, which is a transmembrane receptor
tyrosine kinase
- a mutation in the kit gene leads to constitutive activation and oncogenic signaling in the cell
- GIST tumors express the CD117 antigen, which can be identified by immunohistochemical staining
- the majority of GIST tumors lacking kit mutations have an activating mutation in the platelet-derived
growth factor receptor alpha (PDGFRA)
- Presentation
- some GIST tumors are asymptomatic and are found incidentally at surgery, during endoscopy, or on imaging
- additionally, some GIST tumors present with nonspecific symptoms like early satiety, bloating, or abdominal pain
- bleeding is the most common overt symptom: melena is most common, but frank hematemesis may occur
- rarely, tumor rupture may occur, leading to life-threatening intraperitoneal hemorrhage
- some patients present with obstructive symptoms
- between 15% - 50% of patients may have metastatic disease at presentation
- most common sites of metastasis are the liver, omentum, and peritoneum; lymph node metastases
and extra-abdominal metastases are uncommon (<5%)
- Diagnosis
- Endoscopy
- GIST tumors appear as a smooth, round, submucosal tumor, occasionally with a central ulceration
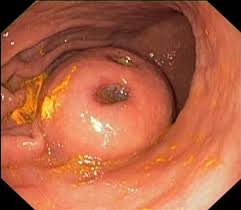
- endoscopic biopsy has a low diagnostic yield, and a tissue diagnosis is not mandatory for
a resectable lesion
- endoscopic ultrasound-guided FNA is more accurate and should be done if neoadjuvant therapy
is being considered or to confirm the diagnosis of metastatic disease
- CT Scan
- used to assess resectability and for the presence of metastatic disease
- GIST tumors appear as a solid, smoothly contoured mass that enhances brightly with IV contrast
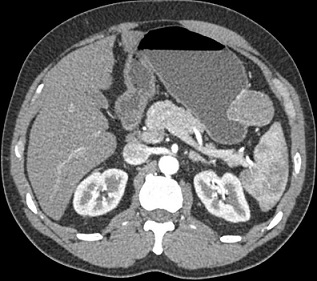
- Prognostic Factors
- there are 3 well established poor prognostic factors: tumor size > 10 cm, mitotic index > 5/HPF, and tumor site
of origin (small intestine)
- mitotic index is the strongest predictor of tumor recurrence
- small bowel GISTs have a higher rate of recurrence than gastric GISTS with similar sizes and mitotic counts
- tumor rupture, either spontaneously or at surgery, has also been verified as an independent risk factor that
negatively affects disease-free survival
- various prognostic models exist that are based on tumor site, size, mitotic index, and presence of rupture
- Treatment
- Surgery
- only potential curative treatment
- all tumors > 2 cm should be resected
- most tumors between 1 and 2 cm should likely be resected, since no GIST tumor can be considered benign
- gastric GISTs < 1 cm can be followed, but any small bowel or colon GIST should be resected,
irrespective of size
- ideal margin of resection is unknown
- goal should be an R0 resection, but there is no data to suggest that an R1 resection needs re-excision
- wedge resection or segmental resection are the most common operations performed
- rarely a total gastrectomy is required
- involved organs should be resected en bloc
- lymphadenectomy is not required since nodal metastasis is rare
- tumor rupture at the time of surgery is associated with increased recurrence
- ~ 50% of patients develop recurrence by 24 months
- Adjuvant Therapy
- imatinib (Gleevec), a tyrosine kinase inhibitor, improves survival in high-risk patients
- eligible patients have tumors > 3 cm or who have a mitotic index > 5/hpf
- length of therapy should be at least 3 years
- imatinib may also be used in a neoadjuvant setting to improve resectability
Gastric Carcinoids
- Incidence
- now classified as neuroendocrine tumors (NETS)
- incidence has been rising
- now make up 8% of GI NETS, compared with 2% in 1950
- role of PPIs in formation of NETs is unclear
- Pathology
- 3 types are recognized
- Type I is associated with chronic atrophic gastritis, low acid output,
and increased gastrin secretion from ECL cells, with a 5-year survival rate > 95%
- Type II is associated with Zollinger-Ellison syndrome, with 5-year survival rates between 70% - 90%
- Type III tumors are large, sporadic lesions not associated with hypergastrinemia, with a 5-year survival < 35%
- Treatment
- treatment requires complete removal
- small lesions may be removed endoscopically
- larger lesions may require wedge resection or partial gastrectomy
- somatostatin analogues are used to treat metastatic disease or carcinoid syndrome
Gastric Lymphoma
- Presentation
- primary gastric lymphoma presents in a similar fashion to that of adenocarcinoma
- anorexia and weight loss are the most common symptoms
- early satiety is common as the gastric wall becomes thickened and non-distensible
- patients may present with complications: bleeding, perforation, obstruction
- systemic symptoms (fever, night sweats) may be present but are rare
- diagnosis is made by endoscopy and biopsy
- Pathology
- most common gastric lymphoma is diffuse large cell B cell lymphoma (55%), followed by MALT lymphoma (40%)
- H.pylori and immunodeficiencies are risk factors for gastric lymphoma
- Evaluation
- bone marrow biopsy, CT chest and abdomen to detect distant disease
- enlarged nodes should be biopsied
- Treatment
- Chemotherapy
- most patients are treated with chemotherapy alone
- risk of perforation with chemotherapy is ~ 5%
- Surgery
- reserved for complications: bleeding, perforation, gastric outlet obstruction,
symptomatic recurrences after treatment failure
- as effective as chemotherapy for limited gastric disease
- H. pylori Eradication
- successful eradication of H.pylori results in remission in 75% of cases of MALT lymphoma
- careful follow up is necessary to document regression
References
- Sabiston, 20th ed., pgs 1213 – 1231
- Cameron, 11th ed., pgs 96 – 103
- UpToDate. Epidemiology, Classification, Clinical Presentation, Prognostic Features, and Diagnostic Work-up
of Gastrointestinal Stromal Tumors (GIST). Morgan MD, Jeffrey. Oct 23, 2018. Pgs 1 – 39

