Gastric Adenocarcinoma
- Epidemiology
- 50 years ago, gastric cancer accounted for 20% to 30% of all cancer deaths in the U.S.
- now it is a relatively rare disease in the United States (~ 27,500 new cases per year with ~ 11,000 deaths)
- the reason for this trend is unknown
- the decline has been in antral tumors of the intestinal type; cancers of the gastric cardia have
been increasing in incidence
- worldwide, gastric cancer is the fourth most common cancer
- worldwide, there is considerable geographic variation in the incidence of gastric cancer: Japan has an
incidence ten times higher than in the U.S.
- Chile, Iceland, Costa Rica have incidences of the disease much higher than in the U.S.
- 2:1 male to female ratio; higher incidence among blacks than whites in the U.S.
- peak incidence is in the sixth and seventh decades of life
- Etiology
- Diet
- gastric cancer appears to be correlated with foods containing high levels of salt, nitrates, and nitrites
- nitrates and nitrites can be converted to carcinogens, the n-nitrosamines
- ascorbic acid can prevent the conversion of nitrites to nitrosamines
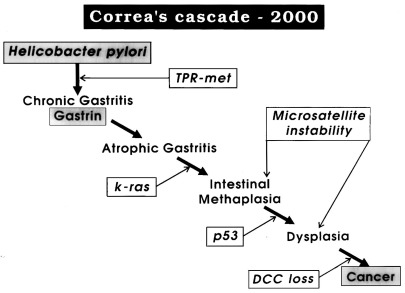
- Helicobacter Pylori
- parallels exist between regional rates of gastric cancer and H. pylori infection
- H. pylori is more associated with the intestinal type cancer than with the diffuse type
- chronic H. pylori infection leads to acute and chronic gastritis, which may progress to chronic
atrophic gastritis with metaplasia, followed by dysplasia
- in addition, chronic atrophic gastritis leads to achlorhydria with consequent anaerobic bacterial overgrowth
- anaerobic organisms are capable of reducing nitrate to nitrite, which induces the synthesis of nitroso compounds
that possess mutagenic potential
- H. pylori also causes production of growth regulatory peptides
- trials aimed at gastric cancer prevention by the treatment of H. pylori infection are currently under way
- Polyps
- adenomatous gastric polyps are rare but carry a high risk for the development of cancer
- the risk is greatest for polyps > 2 cm
- fundic gland polyps are associated with PPI use, and do not appear to have malignant potential
- Previous Gastric Surgery
- gastric surgery for benign conditions increases the risk of gastric cancer twofold to sixfold
- occurs 15 to 20 years following gastric resection and is most commonly associated with a
Billroth II reconstruction
- vagotomy and antrectomy causes hypochlorhydria, leading to bacterial overgrowth
- bacterial overgrowth can lead to increased conversion of nitrites to nitroso compounds,
which can cause metaplasia, dysplasia, and eventually cancer
- the continuous bathing of the gastric mucosa with alkaline secretions is likely another important factor
- Hereditary Risk Factors
- hereditary diffuse gastric cancer results from a gene mutation in E-cadherin, a cell adhesion molecule
- Li-Fraumeni syndrome results from a mutation in p53
- hereditary nonpolyposis colon cancer (Lynch syndrome) is associated with gastric and ovarian cancers
- Peutz-Jegher’s syndrome
- Other Risk Factors
- pernicious anemia results in achlorhydria
- PPIs, which result in achlorhydria and corpus gastritis, have not been shown to cause gastric cancer
- Pathology
- Gross Appearance
- gastric cancers are divided into 4 subtypes based on macroscopic appearance:
polypoid, fungating, ulcerative, and scirrhous (linitis plastic)
- scirrhous tumors infiltrate the entire wall of the stomach and cover a very large surface area
- distribution of tumors: 40% distal, 30% middle, 30% proximal
- Histologic Appearance
- 2 major histologic types: intestinal and diffuse
- additional histologic subtypes include papillary, tubular, mucinous, and signet-ring cell
- Intestinal Type
- localized to the antrum
- arises in the setting of intestinal metaplasia
- tumors have a glandular structure resembling colon cancer
- predominates in high risk areas
- more closely associated with H. pylori than the diffuse type
- Diffuse Type
- arises out of single-cell mutations within normal gastric glands
- not associated with intestinal metaplasia
- poorly differentiated
- associated with more proximal tumors
- seen more often in women and young patients
- stage for stage, has a worse prognosis than the intestinal type
- Methods of Spread
- regional lymphatics
- direct extension into adjacent organs (liver, spleen, pancreas, transverse colon and mesentery)
- systemically via the portal vein
- transperitoneally (ovaries – Krukenberg’s tumor, pelvic cul-de-sac – Blumer’s shelf)
- Early Gastric Cancer (EGC)
- histologically confined to the mucosa and submucosa
- may have lymph node involvement (3%, mucosal tumors; 20% submucosal tumors)
- comprises > 50% of all gastric cancers diagnosed in Japan, largely as a result of mass screening programs
- comprises < 10% of gastric cancers diagnosed in the U.S.
- cure rates are greater than 90% at 5 years
- Pathologic Staging
- Classification by Location
- tumors involving the G-E junction with the tumor epicenter no more than 2 cm into the proximal
stomach are now classified as esophageal cancers
- G-E junction tumors with their epicenter located more than 2 cm into the proximal stomach are
classified as stomach cancers
- TNM Classification
- T Category
- T1a: tumor does not invade the submucosa
- T1b: tumor invades the submucosa
- T2: tumor invades the muscularis propria
- T3: penetrates the subserosal connective tissue but not the serosa
- T4: penetrates the serosa or invades adjacent organs
- N Category
- number of examined nodes affects the accuracy of staging and influences survival
- AJCC guidelines recommend that a minimum of 16 nodes be removed for pathologic evaluation,
and that 30 or more is preferable
- Clinical Manifestations
- Early Gastric Cancer (T1)
- symptoms of early gastric cancer are vague and nonspecific
- may mimic symptoms of gastric ulcer disease
- In the U.S, they are usually found incidentally on EGD for GERD or peptic ulcer disease
- Locally Advanced Gastric Cancer
- weight loss, abdominal pain, and anorexia are the most common symptoms
- nausea and vomiting may occur if distal lesions obstruct the pylorus
- dysphagia is a dominant symptom for cancer of the cardia
- hematemesis is unusual, but anemia and occult blood in the stool are common
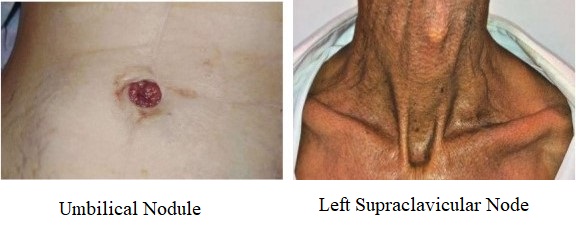
- 10% of patients present with evidence of widespread disease, including hepatomegaly,
ascites, supraclavicular adenopathy (Virchow’s node), ovarian metastases (Krukenberg’s tumor),
Blumer’s shelf, umbilical nodule (Sister Mary Joseph’s nodule)
- Diagnosis and Clinical Staging
- upper endoscopy with biopsy is the most accurate diagnostic tool
- double-contrast barium UGI is a complementary study but it is difficult to
distinguish benign from malignant ulcers by this study
- abdominal/pelvic CT scanning should be done for preoperative staging
- endoscopic ultrasound (EUS) provides accurate data about the depth of tumor
penetration through the stomach wall and showing enlarged perigastric nodes
- EUS is most valuable in distinguishing early gastric cancers from more advanced tumors
- PET-CT is useful for evaluating for distant metastases
- laparoscopy may be used as a staging tool to determine the presence of small liver or intraperitoneal
metastases not seen on CT scan
- Treatment
- Palliation
- unfortunately, many patients with gastric cancer present with advanced disease
(distant metastases or invasion of a major vessel)
- patients with advanced disease who are not bleeding or obstructed should not be explored
- if the patient is bleeding or obstructed, then a palliative resection can be offered to improve
the patient’s quality of life
- it is controversial whether a total gastrectomy is an appropriate palliative intervention
- if, at exploration, an obstructing lesion is not resectable, then a gastrojejunostomy can be performed
- for patients with metastatic obstructing proximal gastric tumors, palliation is best achieved with stents
or endoscopic laser therapy
- radiation and chemotherapy offer little in the way of palliation
- Curative Resection
- surgery is the only potentially curable therapy (R0 resection)
- much controversy exists regarding the extent of gastric resection and the completeness of the lymphadenectomy
- location of the primary tumor is defined as distal third, middle third, upper third, and cardia
- an adequate proximal and distal margin should be 4 to 6 cm from the tumor and should be confirmed
with intraoperative frozen section
- Tumors of the Distal Third
- best managed with subtotal gastrectomy (75%), to include 2 to 3 cm of duodenum
- left and right gastric vessels, left and right gastroepiploic vessels are ligated at their origin
- greater and lesser omentum are also removed
- resection includes the suprapyloric and infrapyloric nodes, as well as the nodes along the greater
and lesser curves
- 16 or more nodes should be removed
- splenectomy is usually not part of the procedure
- reconstruction is by Roux-en-Y or Billroth II gastrojejunostomy
- Billroth I reconstruction is contraindicated because of the risk of local recurrence and
resulting obstruction
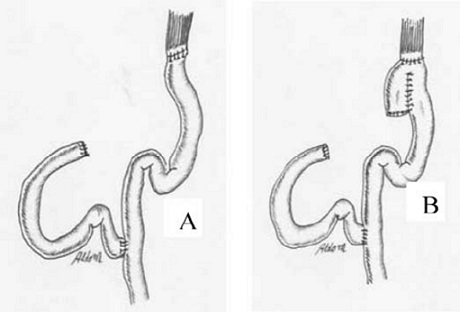
- Tumors of the Middle Third
- usually require a total gastrectomy
- if the tumor is small and well-differentiated, it may be possible to preserve a small cuff of
the upper stomach
- splenectomy and/or distal pancreatectomy for greater curve tumors may be required
- reconstruction is by a Roux-en-Y esophagojejunostomy, with or without the creation of a pouch
- Tumors of the Upper Third
- most surgeons prefer total gastrectomy
- another option is a proximal subtotal gastrectomy, but this operation may leave behind nodes
along the lesser curve
- need a generous esophageal margin with frozen section control
- another option is an esophagogastrectomy through a combined laparotomy-thoracotomy approach
- Tumors of the Cardia and Gastroesophageal Junction
- management is controversial
- surgical options include total gastrectomy and esophagogastrectomy with anastomosis in the chest or neck
- an anastomosis in the chest is prone to reflux and life-threatening leaks
- an anastomosis in the neck can be performed using 3 different incisions: (1) right thoracotomy,
(2) midline laparotomy, (3) left cervical
- alternatively, the thoracic dissection can be performed using the transhiatal approach,
obviating a thoracotomy
- intestinal continuity is reestablished with a left (preferable) or right colon interposition
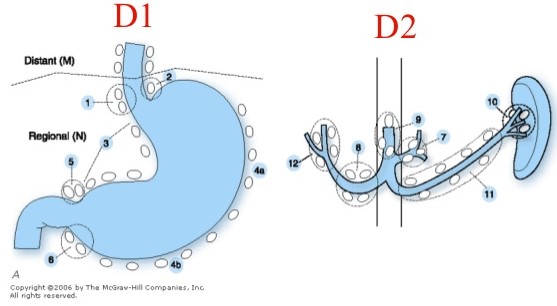
- Extent of Lymphadenectomy
- remains an area of great controversy
- D1: perigastric nodes; has been the standard procedure in the US
- D2: D1 + removal of nodes along the left gastric, hepatic, celiac, and splenic arteries, as well as
splenic hilar nodes
- D3: D1 + D2 + removal of periaortic and porta hepatis nodes
- multiple randomized trials have not shown a survival benefit with D2 versus D1 lymphadenectomy,
or with D3 versus D2 lymphadenectomy
- more recent analysis suggests that a D2 lymphadenectomy may be beneficial if it can be done
without the increased morbidity/mortality of a splenectomy and distal pancreatectomy
- Chemotherapy and Radiation
- Neoadjuvant Chemotherapy
- several recent studies from Europe have shown improved 5-year survival rates over surgery first for
stage II and III disease
- one advantage of preoperative chemotherapy is that adequate postoperative chemotherapy is often limited
by postoperative complications and slow recovery
- Adjuvant Chemotherapy and Radiation
- postoperative chemotherapy alone is indicated for patients who have had a D2 lymphadenectomy
- for patients who have had less than a D2 lymphadenectomy, postoperative chemoradiation is indicated
Gastric Lymphoma
- Presentation
- primary gastric lymphoma presents in a similar fashion to that of adenocarcinoma
- anorexia and weight loss are the most common symptoms
- early satiety is common as the gastric wall becomes thickened and non-distensible
- patients may present with complications: bleeding, perforation, obstruction
- systemic symptoms (fever, night sweats) may be present but are rare
- diagnosis is made by endoscopy and biopsy
- Pathology
- most common gastric lymphoma is diffuse large cell B cell lymphoma (55%), followed by MALT lymphoma (40%)
- H.pylori and immunodeficiencies are risk factors for gastric lymphoma
- Evaluation
- bone marrow biopsy, CT chest and abdomen to detect distant disease
- enlarged nodes should be biopsied
- Treatment
- Chemotherapy
- most patients are treated with chemotherapy alone
- risk of perforation with chemotherapy is ~ 5%
- Surgery
- reserved for complications: bleeding, perforation, gastric outlet obstruction,
symptomatic recurrences after treatment failure
- as effective as chemotherapy for limited gastric disease
- H. pylori Eradication
- successful eradication of H.pylori results in remission in 75% of cases of MALT lymphoma
- careful follow up is necessary to document regression
References
- Sabiston, 20th ed., pgs 1213 – 1231
- Cameron, 11th ed., pgs 87 – 96
- UpToDate. Surgical Management of Gastric Cancer. Mansfield MD, Paul. Aug 02, 2019. Pgs 1 – 49



