Cancer Staging
- Clinical Importance
- staging assists in selection of therapy, estimation of prognosis, evaluation of treatment and comparison of results,
and exchange of information among treatment centers
- staging incorporates tumor size (T), nodal status (N), and distant metastasis (M)
- some tumors (sarcomas) include grade (G)
- Clinical Staging (cTNM)
- based on information obtained before treatment
- tumor size is estimated from physical exam or imaging studies
- nodal status estimated from physical exam or imaging studies (ultrasound, CT scan)
- metastases are evaluated by imaging studies (CT, PET scan, bone scan)
- Pathologic Staging (pTNM)
- includes clinical information and information obtained from the resected specimen and regional nodes
- T1 – T4 indicates increasing tumor size, wall invasion, and involvement of adjacent structures
- N0 = no nodal metastases; N1 - N3 indicates progressive involvement of nodes
- M0 = no distant metastases; M1 = distant metastases
Surgery
- Management of Primary Tumors
- Curative Surgery
- presupposes that the tumor is confined to the organ of origin, or the organ and regional lymph nodes
- patients at high risk for metastatic disease should have a staging workup with appropriate imaging studies,
with the goal being avoidance of an incurable operation
- Margins
- goal of oncologic surgery is to obtain microscopically negative margins at the first operation
- inking of the margins, orientation of the specimen by the surgeon, and immediate frozen section
evaluation of margins can assist in achieving negative margins
- adjuvant radiation and chemotherapy cannot substitute for adequate surgery
- optimal macroscopic and microscopic margin amounts are unknown for most tumor types
- Palliative Surgery
- goal is to alleviate pain, infection, bleeding, obstruction without curative intent
- large operations may be justified if there is no effective non-surgical palliation
- Management of Regional Lymph nodes
- since most solid tumors can metastasize through the lymphatics, many oncologic operations remove
the primary tumor and draining lymphatics en bloc
- node dissections minimize the risk of local recurrence (total mesorectal excision in rectal cancer)
- lymphadenectomy is important for staging and prognosis, and helps guide the use of adjuvant therapy
- for colon cancer and stomach cancer, retrieval of a large number of nodes is associated with improved survival
- Sentinel Node Biopsy (SLN)
- standard of care in breast cancer and melanoma for clinically negative nodes
- defined as the first node to receive drainage from the tumor site
- the SLN is the node most likely to contain metastases, if metastases are present in that nodal basin
- goal is to identify the presence or absence of metastases in the least invasive way
- Lymphatic Mapping
- nodal drainage pattern is determined preoperatively by lymphoscintigraphy
- intraoperatively, use of blue dye and a hand-held gamma probe are used to find the SLNs
- any ‘blue’ node is a SLN
- any node with a gamma count > 10x background is a SLN
- intraoperative detection of the SLN should approach 100% using both the blue dye and gamma
probe techniques
- False Negative SLN Biopsy
- defined as the development of regional node metastases in a patient in whom the SLN was
negative
- may be due to surgical error (removing a non SLN), pathological error, or biologic variation
(metastases bypassing the SLN in favor of a second echelon node)
- reported rates vary between 0% and 11%
- Pathologic Evaluation
- SLN biopsy allows for careful pathologic review of 1 - 2 nodes, which increases the accuracy of nodal staging
- SLN is serially sectioned (bread loafed) and first examined by H + E staining
- if H + E stains are negative, then immunohistochemistry stains are done (S-100, HMB for melanoma; cytokeratin
staining for breast cancer)
- ultrastaging by molecular techniques (RT-PCR) are investigational
- Management of a Positive SLN
- Breast Cancer
- until recently, a positive SLN biopsy mandated a completion axillary node dissection
- a recent ACS multicenter trial demonstrated that in patients with 1 or 2 positive SLNs treated with
breast conservation and systemic therapy, omission of axillary dissection did not result in a
worse outcome
- Melanoma
- trials are underway to determine the role of completion node dissection
- currently, standard management is completion node dissection
- Management of Distant Metastases
- on occasion, patients with metastases to the liver, lung, or brain can be resected for cure
- some tumor types are more amenable to surgical resection than others (colon vs pancreas)
- growth rate of the tumor is also important: patients with a longer disease-free interval have a
higher cure rate after metastasectomy than patients with shorter disease-free intervals
- some surgeons will monitor a potentially resectable patient for several months to see if additional metastases develop
- surgical goal is resection of the metastases with negative margins
- tumor ablation with cryotherapy or radiofrequency ablation is an alternative if tumor location or inadequate hepatic
reserve precludes a safe, negative margin resection
Radiation
- Mechanism of Action
- XRT damages cells by transferring energy and causing ionization of the atoms
- ionization results in double strand DNA breaks
- ionizing radiation is produced by a linear accelerator
- cell viability is determined by the ability of DNA to repair itself
- malignant cells often lack the ability to repair DNA breaks and mutations
- biologic effect of XRT is lessened by hypoxia
- cells in the G2 or M phase are most sensitive to radiation
- Fractionation
- total radiation dose is given in divided doses over 3 – 7 weeks
- allows some time for damaged nonmalignant tissues to repair themselves
- also allows time for cells in G1 and S phases to progress to the more radiosensitive G2 and M phases
- XRT Delivery
- Simulation
- defines the target and any dose-limiting adjacent organs
- simulation is the process of evaluating which beam path will deliver a homogenous dose to the
target and the smallest possible dose to surrounding tissues
- once the best distribution path has been determined, immobilization devices or skin markings are
used to ensure that daily treatments are given in the same way
- Post Op XRT (Adjuvant)
- usually given 3 – 6 weeks after surgery to allow for wound healing
- allows dose modification based on margin status and histology
- surgical contamination of tissue planes may result in a larger volume of normal tissue requiring
irradiation
- post op tumor bed may be relatively hypoxic and more radioresistant
- post op adhesions may increase the risk of small bowel radiation injury in abdominal or pelvic XRT
- usually administered as external beam therapy, but brachytherapy has value in breast cancer
- Brachytherapy
- radiation source is in direct contact with the tissue requiring treatment
- allows delivery of high radiation doses to the tumor bed while reducing to dose to the
surrounding normal tissues
- brachytherapy catheters (MammoSite) are placed during breast cancer surgery or percutaneously soon after
surgery
- a major advantage of the MammoSite catheter is the short treatment duration (3 days)
- Pre-Op XRT (Neoadjuvant)
- has several advantages: may minimize seeding during surgery, allows for smaller treatment fields since the
operative field has not been contaminated
- may also make inoperable tumors operable, or allow for more conservative surgical treatment
- disadvantages include poorer post op wound healing, and inability to give additional doses in cases of close or
positive margins
- often combined with chemotherapy
- Palliative XRT
- valuable in patients with symptomatic bone or brain metastases
- may also be used prophylactically in lytic metastases in weight-bearing bones such as the femur, tibia, humerus
- Side Effects
- increasing XRT dose causes increased tumor control as well as increased damage to normal tissues (therapeutic ratio)
- fractionation allows normal cell healing before the next dose, but requires an increased total dose of radiation to achieve
the same biologic effect
- side effects may be acute (swelling, tissue irritation) or chronic (tissue fibrosis)
- a small increase in secondary malignancies is attributable to XRT
Chemotherapy
- Biologic Basis
- destroys cells by first-order kinetics: each dose kills a constant percentage of cells, not a constant number
of cells
- drugs target rapidly dividing cells
- drugs are either cell-cycle specific or cell-cycle nonspecific
- Alkylating Agents
- cell-cycle nonspecific
- act by cross-linking DNA or damaging DNA, preventing cell division
- cyclophosphamide, cisplatin
- Antitumor Antibiotics
- cell-cycle nonspecific
- interfere with DNA, RNA synthesis
- doxorubicin, bleomycin
- Antimetabolites
- active against cells in S phase
- interfere with normal synthesis of DNA, RNA by substituting for purines or pyrimidines
- methotrexate, azathioprine
- Plant Alkaloids
- block the cell cycle in mitosis by impairing mitotic spindle formation
- vincristine, paclitaxel
- Combination Chemotherapy
- drugs with different mechanisms of action are combined to allow for additive or synergistic effects
- prevents or delays the emergence of drug-resistant cell lines
- offers a broader range of coverage of resistant cell lines in a heterogenous population
- as tumor size increases, so does the likelihood of drug resistance (Goldie-Coldman hypothesis)
- treatment-free interval is kept as short as possible to allow for recovery of the most sensitive normal tissue
- Drug Toxicity
- common side effects include bone marrow suppression, stomatitis or enteritis, and hair loss
- significant toxicities will require dose reduction, but this will greatly limit the antitumor effects (dose reduction
of 20% can be associated with a 50% decrease in cure rate)
- colony-stimulating factors and erythropoietin will help to keep blood counts normal
- Clinical Uses
- Adjuvant Chemotherapy (Post op)
- used in patients at high risk for metastases, but with no evidence of distant disease
- goal is to eliminate micrometastatic disease
- Neoadjuvant Chemotherapy (Pre-op)
- tumor regression may make inoperable tumors operable
- may allow for more conservative surgery (breast conservation, e.g.)
- allows for treatment of micrometastases without the delay of postop recovery
- allows for assessment of clinical and pathological response to treatment – patients who have
an inadequate response may be offered alternative therapies, if available
- postop wound complications are not higher in patients treated with neoadjuvant chemo
- can complicate tumor localization, margin analysis, SLN mapping, pathologic staging
- Routes of Administration
- systemic administration treats micrometastases all over the body, but also causes systemic toxicity
- regional chemotherapy allows targeted organ delivery and minimizes systemic complications
- uses of regional chemotherapy include hepatic artery infusion catheters for colorectal metastases,
limb perfusion for extremity melanoma or sarcoma, intraperitoneal hyperthermic perfusion for pseudomyxoma
peritonei
Hormonal Therapy
- Biologic Basis
- growth of many tumors is under hormonal control
- initial therapy for hormonal control was surgery (oophorectomy, orchiectomy)
- Drugs
- tumor growth can be inhibited by blocking or antagonizing the hormone causing growth (tamoxifen)
- some drugs block the synthesis of the hormone (aromatase inhibitors block the conversion of androgens to
estrogen in postmenopausal women)
- in breast cancer, presence or absence of estrogen and progesterone receptors is used to guide the use of
hormonal therapy
Immunotherapy
- Tumor Antigens
- cancer cells can overexpress or abnormally express a variety of normal cellular proteins that are potentially recognizable
by T cells
- an abnormal gene product (oncogene) could also serve as a good antigen candidate
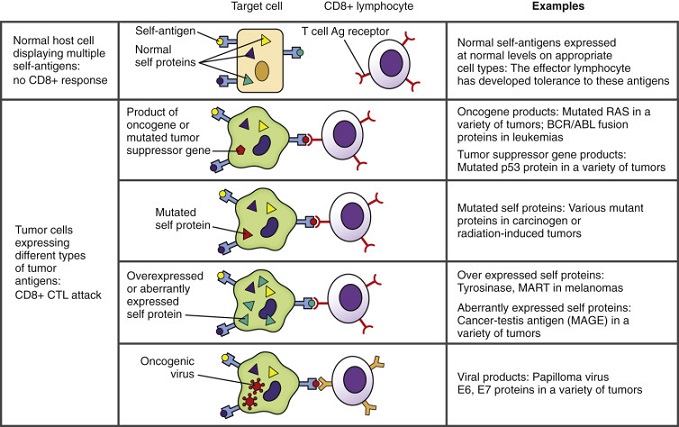
- Tumor Defense Mechanisms
- clinically evident cancers have acquired many defense mechanisms against the immune system
- some tumors lack MHC molecules
- tumors may produce immunosuppressive substances (TGF-β)
- antigen overload: antigens shed from tumor surfaces may bind to circulating antibody and effectors
cells, preventing them from interacting with tumor cells
- antigenic modulation: nonreactive clones of tumor cells replace those destroyed by the immune system
- immune system naturally downregulates itself as a normal protective measure
- induction of tolerance: nonreactivity to antigens may result from high doses of antigen and persistence of antigen
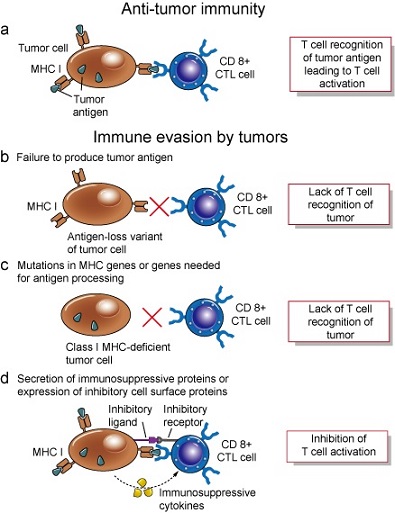
- Evidence for an Immune Response Against Tumors
- frequency of malignant disease is much higher in immunosuppressed patients (AIDS, transplant patients)
- spontaneous regression of metastatic melanoma and renal cell carcinoma is well-described
- 3% - 5% of melanoma patients present with nodal metastases with an unknown primary
- tumor infiltration with lymphocytes in melanoma may be associated with an improved prognosis
- in a small subset of patients, immune therapies can cure patients of widely metastatic melanoma and renal cell carcinoma
- Clinical Immunotherapy
- Nonspecific Immunotherapy
- activates NK cells, macrophages, and lymphocytes
- IL-2 and IFN-α have some efficacy in metastatic melanoma and renal cell carcinoma
- IL-2
- promotes T cell division, B cell growth, and activation of NK cells and monocytes
- has significant toxicities resembling septic shock
- overall response rate is only 15%
- however, a complete and durable tumor response is seen in 4% to 7% of patients
- may be combined with chemotherapeutic agents or other biologic agents
- IFN-α
- antitumor effects include increased expression of MHC class I and class II molecules,
activation of NK cells and macrophages, stimulation of B cells
- approved for use as adjuvant treatment in node positive melanoma – there is a delayed time
to recurrence, but no overall survival benefit
- Vaccines
- earliest strategy was to use allogeneic cultured cancer cells
- autologous tumor vaccines have the advantage of containing antigens relevant to the individual patient,
but they require a large amount of tumor tissue for preparation
- vaccines are usually administered along with nonspecific immune-activating agents
- identification of tumor rejection antigens has made it possible to make antigen-specific vaccinations
- there are multiple studies and clinical trials underway to evaluate different vaccine approaches
- overall, tumor vaccination in patients with metastatic disease has had disappointing results (response rate of 3.6%)
- most human cancer antigens are normal, nonmutated self-proteins that are well tolerated by the immune system
- Why Doesn’t Tumor Vaccination work
- most human cancer antigens are normal, nonmutated self-proteins that the immune system does not react
strongly to
- also, the large tumor burden overwhelms the immune response
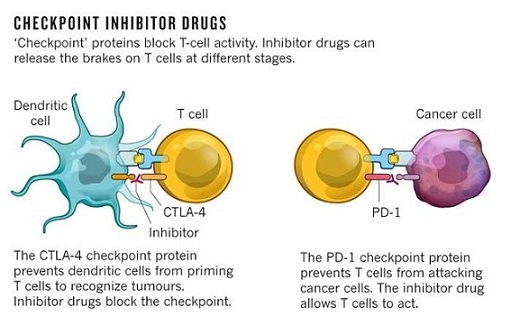
- Immunomodulatory Pathways (Checkpoint Inhibitors
- CTLA-4 and PD-1 are inhibitory receptors on T-cells that serve to downregulate the immune response
(prevents autoimmunity)
- these mechanisms are active in the tumor microenvironment, because of its chronic antigenic stimulation
- some patients treated with a CTLA-4 blocking antibody (ipilimumab) have had durable complete regression of
their metastases
- side effects include autoimmune problems: colitis, dermatitis
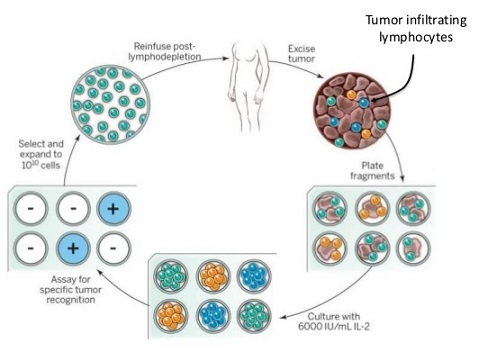
- T-Cell Adoptive Therapy
- T cells are the main effectors of tumor rejection
- fundamental concept is to isolate, expand, and readminister tumor-reactive T cells
- resected metastatic melanoma lesions often contain tumor-infiltrating lymphocytes, which can be activated and
expanded in vitro by adding IL-2 to the culture medium
- systemic high-dose IL-2 is also administered to support TIL survival
- lymphodepletion techniques improve TIL survival in vivo and improve durable response rates
- Monoclonal Antibody Therapy
- fastest growing class of new therapeutic agents in cancer
- initially created from mouse hybridoma technology – mABs were specific but were limited in use by human anti-mouse antibodies
- molecular engineering techniques now allow fully human antibodies to be produced
- Mechanism of Action
- Physical Binding
- mAB binds to a specific tumor antigen
- Herceptin (trastuzumab) blocks signaling through an overexpressed growth factor receptor (Her2/neu)
- Immune system Activation
- mAB directed against a tumor antigen can activate the patient’s immune system to attack the tumor tissue
- Fc region of the antibody can bind to NK cells, phagocytes, and neutrophils, leading to tumor cell
destruction mediated through the ADCC mechanism
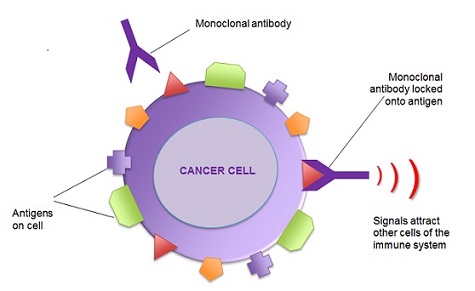
- Unconjugated mABs
- Erbitux (cetuximab) targets the EGFR by binding in a non-activating way, leading to receptor blockade
- Avastin (Bevacizumab) targets VEGF, the ligand of the VEGFR on endothelial cells, inhibiting angiogenesis
and is approved for use in metastatic colorectal cancer
- mABs usually used in combination with chemotherapy
- Conjugated mABs
- mABs conjugated to radionuclides can be used as targeted systemic radiation therapy
- radiation source is delivered to the site of the tumor
- limitation of this approach is poor tumor penetration and bone marrow suppression
References
- Schwartz, 10th ed., pgs 300 – 316
- Sabiston, 20th ed., pgs 705 - 721
- Simmons and Steed, pgs 151 - 158/




