Pleural Anatomy and Physiology
- Anatomy
- the parietal pleura, consisting of mesothelial cells, lines the inner surface of each hemithorax
- at the pulmonary hilum, the parietal pleura becomes invaginated to form the visceral pleura,
which is intimately attached to the lung
- the parietal and visceral surfaces normally are in apposition and lubricated by a thin layer of
serous fluid
- the pleural space is normally only a potential space
- only the parietal pleura has sensory (pain) fibers
- Physiology
- pleural fluid serves as a lubricant, allowing the 2 pleural surfaces to slide past each other during respiration
- normally, between 5 and 10 liters of fluid enters the pleural space each day
- the fluid is produced by the parietal pleura and primarily absorbed by lymphatics in the parietal pleura
- a small imbalance between production and absorption can lead to a pleural effusion
- common etiologies for effusions include increased capillary hydrostatic pressure (congestive heart
failure), decreased oncotic pressure (hypoalbuminemia), increased capillary permeability (sepsis),
and malignant lymphatic obstruction
Pleural Problems
- Pleural Effusions
- Clinical Manifestations
- most common symptom is dyspnea
- many patients are asymptomatic
- Diagnosis
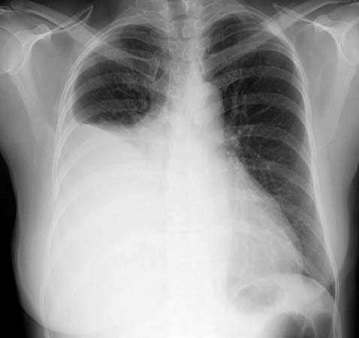
- Chest X-ray
- upright chest x-ray is the initial diagnostic tool
- effusions < 300 cc may not be apparent on the upright chest x-ray
- lateral decubitus films can detect smaller effusions and confirm that the fluid is free-flowing
- Thoracentesis
- mainstay of diagnosis
- distinguishes between transudative and exudative effusions
- positive cytology is diagnostic of malignancy
- positive cultures are diagnostic of empyema
- milky-colored fluid is diagnostic of chylothorax
- elevated amylase levels are suggestive of a sympathetic effusion from pancreatitis, pancreatic pseudocyst,
or pancreatic ascites
- pleural pH < 7.20 strongly suggests bacterial infection
- Video-Assisted Thoracoscopy
- provides excellent visualization of the thoracic cavity
- valuable tool when a biopsy of the pleura or lung is required
- Types of Effusions
- Transudates
- ultrafiltrates of plasma low in total protein (<3.0 g/dL)
- common causes include congestive heart failure, cirrhosis, sepsis
- Exudates
- protein-rich effusions with pleural fluid protein/serum protein > 0.5 or pleural fluid
LDH/serum LDH > 0.6
- more likely to be associated with a diseased pleura
- common causes include malignancy, infection, sympathetic effusions from pancreatitis or a
subphrenic abscess
- Treatment
- Transudative Effusions
- treatment is directed at the underlying condition
- repeat thoracenteses may be required
- occasionally, chest tube placement with or without pleurodesis may be necessary
- Malignant Pleural Effusions
- lung, breast, ovarian, and gastrointestinal cancers are the most common primary tumors responsible
for pleural effusions
- fluid is exudative in character and frequently bloody
- treatment is palliative since median survival is only 3 – 6 months
- Initial Management
- therapeutic thoracentesis determines the symptomatic response to drainage, the ability of
the lung to reexpand completely, and the subsequent rate of reaccumulation
- for some chemoresponsive tumors (breast, ovarian, lymphoma), treatment of the underlying
malignancy may prevent effusion recurrence
- Recurrent Malignant Effusions
- Pleurodesis
- eliminates the pleural space by creating an inflammatory fusion between the
visceral and parietal pleura
- as the first step, the pleural fluid must be completely evacuated and the lung
re-expanded
- talc is now the most common agent used and may be administered at bedside through
a chest tube or at the time of thoracoscopy
- following talc pleurodesis the chest tube is left in place until the output is minimal
- besides talc, other sclerosing agents in use include doxycycline (painful) or
bleomycin (expensive)
- the inflammatory response may cause severe pain in some patients
- success rate is 80% to 90%
- Indwelling Pleural Catheter
- allows for intermittent outpatient drainage
- catheters are typically left in place for 2 – 6 weeks
- spontaneous pleurodesis may occur in ~ 40% of patients
- may also be combined with talc pleurodesis
- serious complications include tract infection, empyema, catheter blockage, and catheter fracture
during removal
- only option for patients with a lung that cannot be reexpanded
- Spontaneous Pneumothorax
- Etiology
- nontraumatic pneumothorax most commonly results from rupture of a pulmonary bleb or bulla
- large leaks can produce a life-threatening tension pneumothorax
- 80% occur in young, tall, thin males without significant pulmonary disease
- in patients over 40, primary pulmonary disease (COPD, emphysema) is usually present
- Clinical Manifestations
- chest pain and dyspnea are the most common findings
- on physical exam, decreased breath sounds and hyperresonance to percussion may be present
- Diagnosis
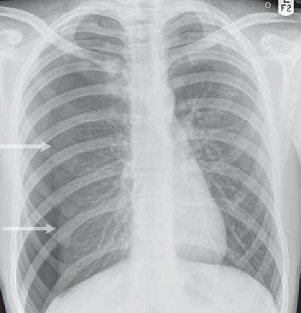
- characteristic x-ray finding is absence of lung markings and a faintly visible line defining
the edge of the lung
- CT scan may identify the cause of the spontaneous pneumothorax
- Treatment
- Nonoperative Treatment
- most spontaneous pneumothoraces can initially be managed with anterior placement of a
small-bore drainage catheter
- progression in size mandates a chest tube
- Tube Thoracostomy
- 20 Fr tube is appropriate for most cases
- tube is placed in the 4th or 5th intercostal space in the midaxillary line and is
directed towards the apex
- most common chest drainage system is the pleur-evac
- tube may also be attached to a one-way Heimlich valve (useful for outpatient management)
- if the air leak is large, more than one chest tube may be necessary to expand the lung
- Operative Treatment
- indicated for a massive air leak with failure of lung reexpansion or for a smaller leak that has
persisted for more than one week
- other indications include recurrent pneumothorax or a first episode in a patient with an occupational
hazard (airplane pilot, diver)
- Video-Assisted Thoracic Surgery (VATS)
- most common operative procedure
- less pain and less postoperative respiratory dysfunction than thoracotomy
- apical blebs are resected with staplers
- pleurodesis is accomplished mechanically by pleural abrasion or chemically by talc
- Thoracotomy
- rarely necessary
- may be done through an axillary incision
- Chylothorax
- Etiology
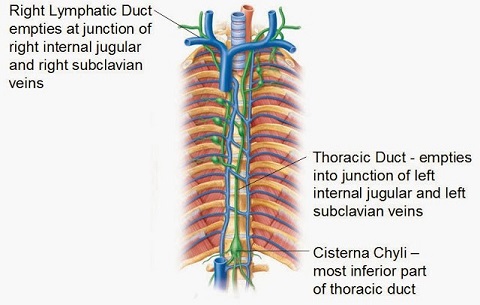
- results from leakage of lymphatic fluid (chyle) from the thoracic duct into the pleural space
- the thoracic duct traverses the mediastinum and enters the venous system at the confluence of the
left internal jugular and subclavian veins
- most chylothoraces are unilateral and on the left side
- most common cause is operative injury (esophagectomy, mediastinal node dissection, neck dissection)
- traumatic chylothorax usually results from penetrating injuries
- most common noninjury cause is malignancy (lymphoma)
- Diagnosis
- aspiration of milky white, odorless fluid is virtually diagnostic
- lymphocyte count, triglyceride level, and the presence of chylomicrons in the fluid can help in
ambiguous cases
- Management
- Nonoperative Management
- goals are to decrease chyle production and keep the lung expanded
- a medium chain triglyceride diet or NPO/TPN are used to reduce chyle production
- a chest tube is required to keep the lung expanded
- nonoperative management should not be tried for more than 7 to 10 days because significant
immunosuppression results from long-term thoracic duct drainage
- Operative Management
- ligation of the thoracic duct at the site of injury is the procedure of choice
- if the site of injury cannot be identified, then the thoracic duct may be ligated at the
diaphragmatic hiatus via a right thoracoscopy or thoracotomy
- Empyema
- infection of the pleural space
- Etiology
- most frequently caused by pneumonia
- other causes include trauma, postoperative, bronchopleural fistula, esophageal perforation or anastomotic
leak, or spread from an intraabdominal source (subphrenic abscess, intrahepatic abscess)
- most common organisms isolated are Staphylococcus, Streptococcus, gram-negative rods (Pseudomonas,
Klebsiella, E. coli), and anaerobes
- Clinical Manifestations and Diagnosis
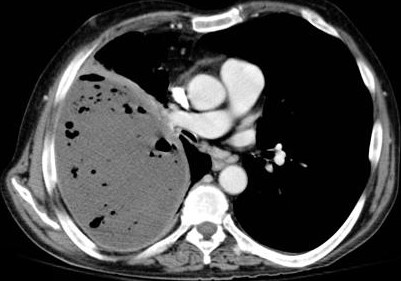
- patients are febrile and have a pleural effusion on chest x-ray or CT scan
- thoracentesis with Gram stain and culture of the fluid confirms the diagnosis
- pleural fluid with a pH < 7.20 and glucose < 40 mg/dL also strongly suggests the diagnosis
- CT scan may be necessary to rule out other pathology such as a lung abscess
- Treatment
- principles are appropriate antibiotics, obliteration of the empyema space by lung reexpansion,
and complete dependent drainage
- Thoracentesis
- may be used to make the diagnosis, but is insufficient treatment because the empyema space is
not obliterated
- Thoracostomy Tube
- usually the first treatment chosen
- must use a large tube in order to evacuate the thick, viscous material
- fibrinolytics and DNase may be used to break up loculations
- should be placed in the most dependent part of the empyema cavity
- if a small space persists after closed-tube drainage, the drainage can be converted to
open drainage by cutting the tube off at the skin and allowing it to drain into dressings
- Open Drainage with Rib Resection
- allows for evacuation of pus and adequate breakup of loculations and adhesions
- if the cavity is mature it can be marsupialized – this facilitates dressing changes and irrigation
- Decortication
- requires a thoracoscopy or formal thoracotomy
- empyema space is evacuated under direct vision and the inflammatory peel is completely
removed from the visceral pleura
- goal is to allow lung reexpansion
- chest tubes are placed in the most dependent position
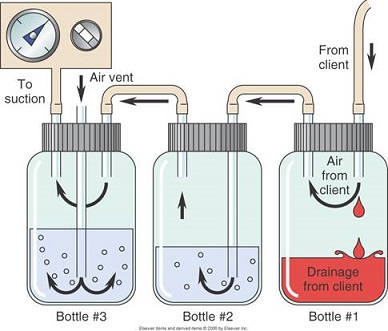
- Chest Tube Mechanics
- the tube is connected to a collection device that consists of 3 separate but interconnected
bottles or chambers
- must be placed below the level of the chest for gravity drainage
- Bottle #1
- connects directly to the chest tube
- used for fluid collection
- air flows into the next bottle
- Bottle #2
- air flows into the water at the bottom of the bottle
- bubbles indicate an air leak
- functions as a one-way valve: air can escape the chest on exhalation but cannot get
back in during inhalation
- the water seal chamber must be filled to 2 cm
- Bottle #3
- manometer allows you to set a defined amount of suction through the chest tube
References
- Schwartz, 10th ed., pgs 680 - 690
- Sabiston, 20th ed., pgs 1601 – 1613
- UpToDate. Management of Malignant Pleural Effusions. John E. Heffner, MD. May 05, 2020. Pgs 1 – 19
- How a Chest Tube Drainage System Works. Jean Sun, MD. Sinaiem.org




