Immunobiology
- Innate and Acquired Immunity
- Innate Immunity
- older, nonspecific immune system that is broadly reactive against common components of
pathogenic organisms (e.g. endotoxin)
- also responsible for identifying altered or damaged tissue
- effector cells include macrophages, monocytes, neutrophils, natural killer cells
- Acquired Immunity
- purpose is specific recognition of foreign antigen and elimination of nonself
- another purpose is to respond quickly to prior antigenic challenges
- based on antigen receptors formed by germline rearrangement, which leads to highly specific
binding interactions
- mediated by T cells and B cells
- each T cell or B cell recognizes only one particular antigen
- 109 different clones of T cells and B cells exist in each individual
- T Cells
- protect the cells of the body against alterations by mutation or viral infection
(cellular immunity)
- T cell receptors only recognize fragments of peptide antigens bound to MHC molecules
- 2 main types of T cells: CD4 (T helper) and CD8 (T cytotoxic)
- CD4 T cells recognize antigen bound to class II MHC molecules, which are found on
antigen-presenting cells such as dendritic cells, macrophages, B cells
- CD8 cells recognize antigen bound to Class I MHC molecules, which exist on all
nucleated cells
- B Cells
- provide protection against extracellular infectious organisms and foreign material
- recognize antigen in its native unprocessed state
- secrete antibodies to bind foreign molecules
- material bound by antibody is then marked for destruction by phagocytic cells
- bound antibody also activates a destructive enzymatic cascade (complement system)
- Amplification of the Immune Response
- mediated by cytokines
- IL-2 is produced by activated T cells and stimulates proliferation of activated
T cells and B cells
- Major Histocompatibility Complex
- called the HLA locus in humans, and is located on chromosome 6
- produces cell surface proteins that are the principal antigenic determinants of graft rejection
- antigens are grouped into 2 classes: class I and class II
- many different alleles exist for each class I and class II molecule gene (polymorphism)
- likelihood of any two random individuals expressing the same MHC antigens is extremely small
- Class I Molecules
- occur on all nucleated cells in contact with blood
- 3 major class I antigens: HLA-A, HLA-B, HLA-C
- functional part of the class I molecule is the peptide binding groove, which is occupied by
a native peptide
- T cells are able to inspect and approve of ongoing protein synthesis
- T cells bind but do not activate when encountering self MHC molecules presenting self-peptides
- alteration in peptide content (e.g. by viral peptide synthesis or mutation) causes activation
- in transplantation, T cells may be activated directly by the donor’s HLA molecules, or
indirectly by APCs that have processed and presented the foreign antigen
- class I molecules are bound only by cytotoxic CD8 cells
- Class II Molecules
- located on specialized immune cells (antigen-presenting cells, macrophages, dendritic cells, B cells)
and endothelial cells
- consists of an alpha chain and a beta chain
- 3 major class II antigens: HLA-DR, HLA-DP, HLA-DQ
- functional part is the peptide-binding groove between the two polypeptide chains
- proteins engulfed by phagocytic cells are degraded and then associated with class II molecules
- this allows circulating foreign proteins to be presented to T cells
- CD4 cells (T helper) bind class II molecules
- CD4 cells are able to activate CD8 cells and B cells
- when an inappropriate peptide is detected, CD4 cells release cytokines to recruit CD8 cells
into the area
- B cells release antibody to bind the offending peptide and aid in its clearance by phagocytic
cells and the complement cascade
- in transplants, an abnormal peptide or the foreign class II molecule itself can lead to
T cell activation
- Genetics of HLA
- unit of inheritance is the haplotype, which consists of one copy of chromosome 6 and one
copy of each class I and class II locus
- probability of a sibling being HLA-identical is 25%, haploidentical is 50%, and completely
nonidentical is 25%
- parents are haploidentical with their children
- HLA Matching
- matching the recipient and donor as closely as possible with regard to HLA type reduces the
risk of acute rejection
- as immunosuppression has improved, the importance of HLA-matching has decreased
- Tissue Typing
- the more antigenic the graft, the more vigorous the rejection response
- antigenicity of the graft is determined by the degree of genetic disparity
- historically, HLA disparity has been defined with the use of two biologic assays:
the lymphocytotoxicity assay and the mixed lymphocyte reaction (MLC)
- molecular techniques now exist for precise genotyping of an individual’s HLA
Transplant Antigen Recognition
- T Cell Activation
- T cells can respond directly to intact allo-MHC molecules on the surface of the donor tissue
- T cells may also encounter antigen-presenting cells that have phagocytosed fragmented allograft
tissues and processed the antigens for expression with self-MHC
- understanding T cell activation is the key to understanding the rational use of immunosuppressive
agents to prevent acute rejection
- T Cell Binding
- a single interaction with an MHC molecule is not sufficient to cause T cell activation
- T cell must register multiple receptor/ligand interactions with the same antigen before a
threshold of activation is reached (signal 1) - transient encounters are not sufficient
- additional costimulatory pathways (signal 2) are required for T cell activation – additional T cell
receptors must bind to specific ligands on the antigen presenting cell surface
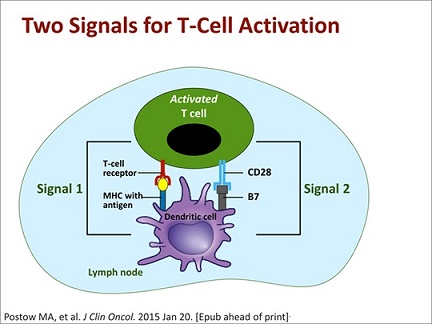
- Intracellular Signaling
- repetitive binding signals eventually result in the T cell receptor becoming internalized,
where it binds to immunophilin in the cytoplasm
- immunophilin stimulates calcineurin, which in turn activates the cytokine transcription factor NF-AT
- activated NF-AT then translocates to the nucleus where it initiates transcription of IL-2
- IL-2 is then released and binds to the T cell in an autocrine loop
- IL-2 binding stimulates the T cell to undergo cell division and replication
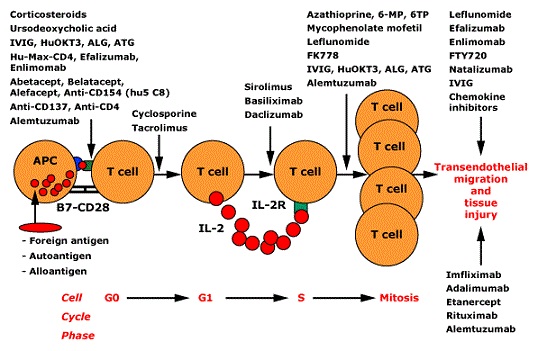
- T Cell Amplification
- once activation occurs, cytokines, particularly IL-2 and interferon-γ, recruit other T cells into
the response
- B cell activation also is mediated through cytokine secretion
- cytokines are responsible for the systemic symptoms of fever and malaise associated with severe graft
rejection
Clinical Rejection Syndromes
- Hyperacute Rejection
- caused by presensitization of the recipient to a donor antigen
- develops within the first minutes to hours following graft reperfusion
- exposure is usually from a prior transplant, transfusion, or pregnancy
- preformed antibodies bind to donor endothelial cells, initiating complement-mediated lysis and a
procoagulant state, resulting in immediate graft thrombosis
- no treatment exists
- can be avoided in 99.5% of transplants by proper ABO matching and a negative transplant antigen
crossmatch assay
- Acute Rejection
- caused primarily by T cells
- usually occurs within the first 6 months after transplant
- inevitable result of allotransplants unless immunosuppression against T cells is used
- to initiate acute rejection, T cells bind donor antigen directly or after phagocytosis of donor tissue
- this leads to T cell activation and massive infiltration of the graft by T cells, leading to
organ destruction
- incidence of acute rejection declines with decreasing MHC disparity
- only kidneys can be preserved long enough to allow organ allocation to the recipient to be the most
closely matched to the MHC of the donor
- treatment of acute rejection leads to successful restoration of graft function in 90 to 95 percent
of cases
- prompt recognition is imperative, and monitoring must be intense, especially during the first year
after transplant
- unexplained graft dysfunction should prompt biopsy and evaluation for the lymphocytic infiltration
and parenchymal necrosis characteristic of acute rejection
- liver acute rejection is also characterized by eosinophilic infiltration of the graft
- Chronic Rejection
- poorly understood
- onset is insidious, occurring over months to years
- it is untreatable, since the pathophysiology is undefined
- increased immunosuppression is not effective in reversing or retarding the progression
- distinguished from acute rejection by biopsy (parenchymal replacement by fibrous tissue with a
relatively sparse lymphocytic infiltrate)
- requires retransplantation
Immunosuppression
- General Principles
- no immunosuppressive intervention is allograft-specific
- all interventions do so at the expense of a vital defense network
- rational, selective use of several immunosuppressive agents acting through different synergistic
pathways is required to successfully prevent rejection without completely removing the body’s
defenses
- immunosuppression is extremely intense in the early postop period (induction immunosuppression)
- induction therapy involves deletion of the T cell response completely and cannot be maintained
indefinitely without lethal consequences
- maintenance immunosuppression is used to prevent acute rejection for the life of the patient
- rescue agents are immunosuppressants used to reverse an acute rejection episode
- Corticosteroids
- a mainstay of virtually all immunosuppression induction and maintenance regimens
- in combination with other agents, they significantly improve graft survival
- high doses are used as a rescue agent to treat acute rejection
- mechanism of action has not been completely elucidated
- functional effect is to depress all T cell responses
- total blood lymphocyte count decreases within 6 hours of corticosteroid administration
- steroids inhibit the production of T cell proinflammatory cytokines and so prevent
the primary mechanism by which lymphocytes amplify their responsiveness
- steroids inhibit both chemotaxis and phagocytosis by macrophages and neutrophils
- many acute side effects (glucose intolerance, poor wound healing, salt and water
retention, CNS effects)
- many chronic side effects (Cushing’s syndrome, cataracts, muscle wasting, osteoporosis)
- current trend in transplantation is to lower the dose of steroids used and add other
immunosuppressive agents
- patients who have survived a year without a rejection episode may be considered for
withdrawal of steroids
- Antiproliferative Agents
- Azathioprine
- purine analog
- cleaved in the liver to form the active compound, 6-mercaptopurine
- prevents RNA and DNA synthesis
- inhibits the replication of T and B cells
- major side effects include bone marrow suppression (leukopenia) and liver
toxicity
- has largely been replaced by mycophenolate mofetil
- Mycophenolate Mofetil (MMF)
- more specific purine antimetabolite
- prevents a critical step in RNA and DNA synthesis
- MMF exploits a critical difference between lymphocytes and other cells to
produce relatively selective immunosuppressive effects
- blocks the proliferative response of both T cells and B cells
- causes less bone marrow suppression than azathioprine
- is not nephrotoxic or hepatotoxic, but GI side effects (diarrhea, nausea,
bloating) can be disabling
- teratogenic in pregnant females
- Calcineurin Inhibitors
- Cyclosporine
- produced by a fungus
- its introduction in 1983 revolutionized transplantation, especially cardiac
and liver transplantation
- T cell specific
- binds with high affinity to cyclophilin in the cell cytoplasm, which inhibits calcineurin and
prevents it from activating the transcription-regulating factor NF-AT
- this prevents transcription of the IL-2 gene and other genes critical for
T cell activation
- works solely as a maintenance agent; it is ineffective as a rescue agent
- most significant side effect is nephrotoxicity
- other side effects include hypertension, tremor and other neurotoxicities,
hyperkalemia, hirsutism, hepatotoxicity
- Tacrolimus (FK-506)
- produced by a fungus
- like cyclosporine, it blocks the effects of NF-AT, prevents cytokine
transcription, and arrests T cell activation
- has a different intracellular target than cyclosporine (FK-binding protein)
and is one hundred times more potent in inhibiting IL-2 production
- main role is as a maintenance agent, but it has shown promise as a rescue agent
- side effect profile is similar to that of cyclosporine with regard to renal
and hepatic toxicity
- extremely effective in liver transplantation and has largely replaced cyclosporine
- high rate of posttransplant lymphoproliferative disorders in children
- Mammalian Target of Rapamycin Inhibitors
- Sirolimus (Rapamycin)
- structurally similar to tacrolimus, but does not inhibit calcineurin
- impairs signal transduction by the IL-2 receptor, thus inhibiting T cell and B cell
proliferation
- nephrotoxicity and neurotoxicity have not been observed
- hepatic artery thrombosis following liver transplant is a major side effect
- Monoclonal Antibodies
- Antilymphocyte Globulin (ALG)
- polyclonal antibodies produced by injecting human thymocytes into different species (horse, rabbit)
- T cells and B cells are eliminated through complement-mediated lysis and opsonin-induced
phagocytosis
- most commonly used for induction of immunosuppression or treatment of steroid-resistant rejection
- used in kidney, kidney/pancreas, and intestinal transplants, but rarely in liver transplants
- severe thrombocytopenia is a major side effect
- causes an increase in viral reactivation and viral infections (CMV, EBV)
- chills, fever, skin rash occur in 15 to 20 percent of patients - these can be
ameliorated by pretreatment with steroids, antihistamines, and antipyretics
- Anti-IL2 Receptor Antibodies (basiliximab)
- blocks IL2 binding to its receptor
- used during induction
- Inhibition of T Cell Activation
- Belatacept
- blocks T cell costimulation pathways
- has an increased risk of posttransplant lymphoproliferative disorder,
especially in recipients who are EBV-seronegative pretransplant
- given monthly by IV
- Complications of Immunosuppression
- Infection
- most common cause of mortality in transplant patients
- most infections are by opportunistic organisms
- Candida and Aspergillus species are the most common cause of fungal infections
- Pneumocystis Carinii, a protozoan, is a frequent cause of pulmonary infection
- Cytomegalovirus is the most common viral offender
- Malignancy
- incidence of virally-mediated tumors is significantly increased in transplant patients
- rate not high enough to contraindicate transplantation
- incidence of squamous cell skin cancers, Kaposi’s sarcoma, non-Hodgkin’s lymphoma, cancer of
the liver, anus, and cervix are five times as frequent as compared to the general population
References
- Schwartz, 10th ed., pgs 321 - 334
- Sabiston, 20th ed., pgs 598 - 630
- UpToDate. Transplant Immunobiology. John Vela, MD, FACP, FRCP. March 2021. Pgs 1 – 38
- UpToDate. Liver Transplantation in Adults. Norman L. Sussman, MD, John M. Vierling, MD, FACP. Oct 07, 2020. Pgs 1 – 42

