Abdominal Aortic Aneurysms and Aortic Dissections
Abdominal Aortic Aneurysms (AAAs)
- Etiology
- abdominal aorta > 3.0 cm in maximal diameter is considered aneurysmal
- majority of AAAs are fusiform in shape and arise below the level of the renal arteries
- major risk factors include age, male sex, and tobacco use
- definite familial tendency exists
- pathologic examination usually shows inflammation and degeneration of the arterial wall
- loss of elastic tissue is a critical factor
- an imbalance may exist between 2 enzymes important in the metabolism of elastin, elastase
(degradation) and α1-antitrypsin (synthesis)
- inherited connective tissue disorders (Marfan’s syndrome and type IV Ehlers-Danlos syndrome) are associated
with a high incidence of arterial aneurysms
- Risk of Rupture
- AAAs < 4 cm have a very low risk of rupture
- AAAs between 5 and 6 cm have an estimated yearly rupture risk between 3% - 15%
- AAAs > 8 cm have a 30% - 50% risk of rupture/year
- rate of growth > 5 mm in 6 months or 1 cm per year is also a risk factor for rupture
- women also have a higher rate of rupture
- symptomatic AAAs have a higher rate of rupture than asymptomatic AAAs
- Clinical Manifestations
- most are AAAs are asymptomatic and are discovered as a pulsatile periumbilical mass on routine physical exam
or are found incidentally during abdominal imaging studies for other reasons
- abdominal symptoms range from vague abdominal pain to excruciating flank or back pain
- unusual presentations include aortocaval fistula, ureteral obstruction, thrombosis, or embolization (limb ischemia)
- Diagnostic Studies
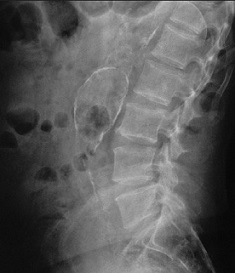
- Plain Films
- calcification of the aneurysm wall is often incidentally observed, especially on the lateral view
- Ultrasound
- accurate, safe, low cost study
- study may be limited in obese patients or if excessive bowel gas is present
- the proximal and distal extent of disease may be impossible to discern
- most valuable as a screening tool or following the size of known aneurysms
- misses 50% of ruptures, so not very useful in symptomatic patients
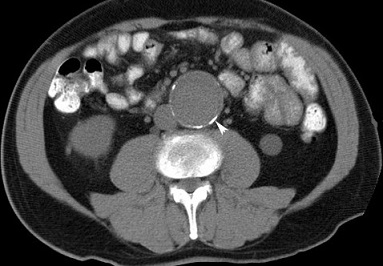
- CT and CTA
- accurately studies the entire aorta
- detects vessel calcification, thrombus, concurrent arterial occlusive disease
- useful for showing the relationship between the renal arteries and the proximal cuff
- extremely valuable in evaluating patients with symptoms
- drawbacks include high radiation exposure and the use of iodinated contrast agents
- MRI and MRA
- typically used if there a contraindication to CT (kidney disease)
- does not detect vessel calcification, which can be important in operative planning
- Medical Treatment
- no drug is currently indicated for reducing AAA enlargement
- medical management is primarily indicated to reduce the risk of a future MI or stroke
- beta blockers are often used to control blood pressure and to reduce aortic wall stress
- statins may be associated with reduced rates of AAA enlargement
- smoking cessation offers the most benefits in reducing the rate of aneurysm expansion and in improving
perioperative morbidity and mortality
- Elective Surgical Treatment
- intervention is recommended when the risk of rupture is greater than the risk of the procedure
- endovascular approaches (EVAR) comprise the majority of procedures performed (80%)
- Indications for Repair
- aneurysms > 5.5 cm in maximal diameter for males, 5.0 cm in females
- > 5 mm of growth in 6 months or > 1 cm of growth in 12 months
- saccular aneurysms
- symptomatic aneurysms
- coexistent aneurysm or peripheral artery disease requiring repair
- Preoperative Evaluation
- important medical comorbidities such as CAD, CRF, diabetes, COPD, peripheral vascular disease must be
identified and optimized
- Coronary Artery Disease
- CAD is the primary cause of morbidity and mortality after open or endovascular AAA repair
- at least 2/3 of AAA patients have clinically significant CAD
- patients with symptomatic CAD with require a full cardiac evaluation, and may require cardiac angiography
and immediate revascularization
- asymptomatic patients with significant CAD risk factors should also undergo echocardiography and stress
testing – evidence of ischemia would mandate angiography
- asymptomatic patients with a functional capacity < 4 METS will also require a full cardiac workup
- asymptomatic, low-risk patients with a normal EKG do not require any further workup
- AAA repair can follow cardiac stenting procedures almost immediately, and 4 – 6 weeks after CABG
- Chronic Renal Insufficiency
- coexistent renal artery occlusive disease is present in 20% to 38% of patients with AAA
- open repair or endovascular repair can also worsen preexisting renal disease
- correction of renal artery occlusive disease should be performed at the time of open AAA repair or EVAR
- to avoid worsening kidney function during the procedure, adequate hydration, avoidance of
hypotension, and discontinuation of ACE inhibitors and angiotensin receptor blockers is recommended
- COPD
- increased risk of prolonged ventilator support after surgery
- ABG, pulmonary function tests are recommended in patients with poor pulmonary function
- some patients will benefit from bronchodilators
- smoking cessation for more than 2 weeks before the procedure is likely beneficial
- Preoperative Imaging
- necessary for determining the best approach – open or EVAR
- anatomic variants that influence approach and technique will be detected – retroaortic left renal
vein, horseshoe kidney, variant inferior vena cava
- demonstrating vascular calcifications allows the surgeon to assess the feasibility of clamping the
aorta and iliacs at various levels
- Open Repair
- Indications
- short aneurysm neck (< 1.5 cm)
- significant angulation (> 60 degrees) of the neck
- suprarenal aneurysm
- young patients – because of the uncertainty about long-term endograft durability and the risk of
late rupture
- endovascular capabilities are not available
- Patient Preparation
- bowel preparation is prudent
- epidural catheters reduce the amount of anesthesia required, allow for prompt extubation,
and control pain in the perioperative period
- Swan-Ganz catheters or intraoperative transesophageal echocardiography are used in patients
with a significant cardiac history
- cell savers reduce the need for banked blood
- perioperative antibiotics effective against S. aureus should be given
- Surgical Approaches
- AAA repair may be performed through a transabdominal or retroperitoneal approach
- Transperitoneal Approach
- necessary when access to the right renal artery is required, when exposure of the right
internal and external iliac arteries is required, and when concomitant abdominal
procedures must be performed
- disadvantages include a longer ileus, more insensible fluid losses, and more difficult proximal
control for juxta renal and suprarenal AAAs
- Retroperitoneal Approach
- useful when there are extensive intraperitoneal adhesions, or when gastrointestinal or urinary
stomas are present
- also valuable when extensive suprarenal exposure is required, and when the left kidney must be
revascularized
- disadvantages include poor access to the right iliac and right renal arteries, and inability to fully
explore the abdomen
- may also be associated with more chronic pain, wound problems, and incisional hernias
- patient is positioned in the right lateral decubitus position
- Operative Conduct
- patient is heparinized (100 U/kg) after the aorta has been exposed
- iliac arteries are clamped first to prevent embolization, followed by the proximal aorta
- aneurysm is opened longitudinally, and thrombus removed
- lumbar artery backbleeding is controlled with suture ligation
- inferior mesenteric artery is also usually suture-ligated because it is often chronically occluded
- if there is poor back-bleeding from the IMA, it should be reimplanted into the graft
- a straight tube graft is used unless there is involvement of the iliac arteries, in which case a
bifurcation graft is chosen
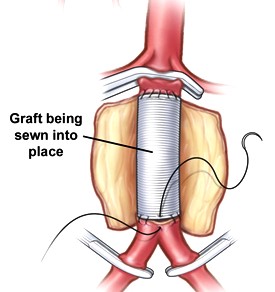
- the aorta is not transected, and the posterior wall of the graft is sewn from within the aneurysm sac
- right before the completion of the anastomosis, the aortic clamp should be removed temporarily to flush
out any debris that might embolize distally
- after the anastomoses are complete, the wall of the aneurysm is sutured over the graft to prevent
intestinal adherence to the graft
- Complications
- Cardiac Complications
- open repair has a 30-day operative mortality rate of 4% to 5%, with most deaths caused by MIs,
CHF, or arrythmias
- prevention starts with an adequate preoperative risk assessment
- in the perioperative period, meticulous control of heart rate, blood pressure, and cardiac
filling pressures are necessary
- Ischemic Colitis
- most commonly involves the sigmoid colon
- much more common after surgery for a ruptured AAA (20% - 40%)
- usually occurs within the first 3 days postop
- symptoms include left lower quadrant pain and tenderness, bloody diarrhea, fever,
tachycardia
- initial diagnostic test is proctoscopy or flexible sigmoidoscopy
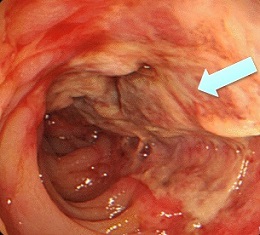
- initial management is with IV antibiotics, fluid resuscitation and maximization of cardiac
output, bowel rest
- patients with peritonitis, or those who do not improve with conservative management, will
require a sigmoid colectomy
- a primary anastomosis is not recommended because a leak may contaminate the aortic graft
- Lower Extremity Ischemia
- usually due to embolization of debris from the aneurysm sac
- prevention is the best treatment: careful dissection of the aorta, careful clamping of the
iliacs, flushing of the vessels, and sequential clamp removal
- thrombosis is a less common cause of ischemia, and management may require thrombectomy,
anticoagulation, and revision of the distal limb
- Other Complications
- ureteral injury occurs in < 1% of cases
- sexual dysfunction (impotence, retrograde ejaculation) occurs in 10% to 20%
- paraplegia occurs in < 1% of cases, but the incidence is much higher
(1.4%) after a ruptured AAA
- graft infection and aortoenteric fistula are late complications and occur in less than 1% of cases
- Endovascular Repair (EVAR)
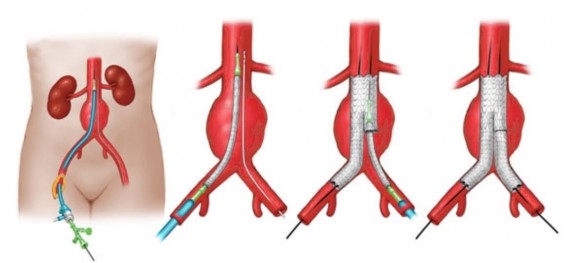
- Overview
- 75% to 80% of elective AAA repairs are performed with endovascular techniques
- EVAR is associated with a shorter length of stay and a decreased 30-day mortality, when compared to
open AAA repair
- EVAR does not improve quality of life beyond 3 months or survival beyond 2 years
- EVAR is associated with a higher rate of re-intervention (which is usually endovascular) than the open procedure
- EVAR is also associated with late AAA rupture
- Patient Selection
- success of EVAR depends heavily on appropriate patient selection
- requirements for EVAR include an appropriate aortic neck, a distal sealing zone, and a suitable
path for the endograft to be placed through
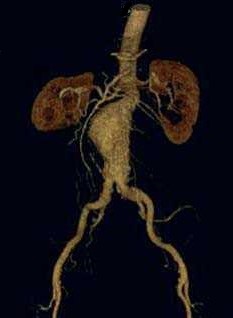
- CTA with reformatted 3-dimensional reconstructions is required to assess the suitability of the anatomy for EVAR
- Aortic Neck Anatomy
- aortic neck is the aorta above the aneurysm and below the renal arteries
- this is the site of proximal fixation of the device
- a circumferential seal must be obtained in this area to prevent blood leakage into the
aneurysm sac
- the aortic neck should be at least 15 mm in length, and its diameter should be less than
32 mm
- angulation of the neck in the vertical plane also needs to be less than 60 degrees to
prevent endoleaks, kinking, and stent migration
- additional factors that can prevent an effective seal and fixation include severe aortic
wall calcification and circumferential thrombus
- Iliofemoral Anatomy
- presence of thrombus, calcifications, and tortuosity can prevent obtaining a distal seal
- most endograft systems require a 15 mm segment of suitable iliac artery
- largest iliac diameter that can be treated is 25 mm
- Complications
- Access Complications
- occur in 3% of cases
- hematoma, pseudoaneurysm, arterial occlusion or dissection
- iliac artery rupture or transection
- Endoleaks
- defined as the persistence of blood flow outside of the endograft within the aneurysm sac
- systemic circulation in the aneurysm sac results in aneurysm sac expansion and potential rupture
- endoleaks are the most common cause of secondary interventions and aneurysm-related morbidity
following EVAR
- Type I
- results from an inadequate seal at the proximal aortic (Ia) or distal iliac (Ib)
attachment sites
- can result from a size mismatch between the graft and vessel, severe aortic neck
angulation, or a heavily calcified aortic wall
- usually detected by angiography immediately after deployment
- requires immediate repair since these leaks result in growth and potential rupture
of the aneurysm sac
- Type II
- most common type of endoleak (10% - 20%)
- occurs from retrograde filling of the aneurysm sac from a patent lumbar artery or IMA
- management consists of close observation, since many will resolve spontaneously and
the risk of rupture is low
- Type III
- caused by defects in the fabric of the graft at the junction of modular components
- treatment consists of placing covered stents to exclude the aneurysm sac from systemic pressure
- Type IV
- caused by leaking of blood between the interstices of the graft fabric
- often resolves after anticoagulation is reversed
- if the leak persists, the endograft may need to be relined
- Type V (Endotension)
- persistent growth of the aneurysm sac in the absence of a detectable leak
- exact etiology is unknown, but may result from an undetected endoleak from one of
the other types
- must be followed closely because of the risk of rupture
- Iliac Limb Thrombosis
- occurs in 11% of cases within 6 months of EVAR
- ipsilateral buttock and/or limb claudication are typical symptoms
- symptomatic patients require an attempt at thrombolysis or a femoral-femoral bypass
- Ruptured AAAs
- overall mortality approaches 90%
- mortality rate of patients who reach the hospital alive is > 50%
- Diagnosis
- classic triad consists of sudden, severe abdominal, back, or flank pain, hypotension, and a palpable
periumbilical mass
- if the hematoma is contained, the initial symptoms may be surprisingly mild
- differential diagnosis includes: perforated viscus, biliary disease, pancreatitis, kidney stones,
acute myocardial infarction
- if the patient is stable, then a CT scan will confirm the diagnosis and help with the operative planning
and approach
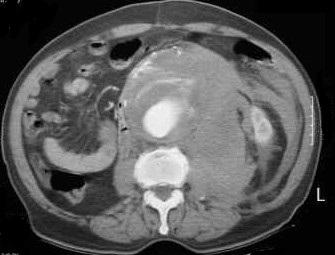
- Resuscitation
- patient should be approached like a trauma patient
- large-bore IVs should be inserted, and blood transfused as necessary
- preventing hypothermia is critical: all blood and fluids should be warmed
- overresuscitation should be avoided
- resuscitation goal is a conscious patient with systolic pressures in the 80 to 100 mm Hg range
(permissive hypotension)
- operation should not be delayed by resuscitation: once the diagnosis is made, the patient should be
brought to the OR
- Aortic Control
- percutaneous placement of an occlusive balloon in the suprarenal aorta will gain proximal control in
most patients
- aortic control via a left thoracotomy should be performed if an occlusive balloon is not available and
the abdomen is expected to be hostile
- Open Repair
- patient should be prepped and draped before the induction of general anesthesia
- open or retroperitoneal approaches are possible
- after the abdomen is opened, if an occlusion balloon has not been placed, the supraceliac aorta
can be compressed manually with a retractor, allowing the anesthesiologist time for resuscitation
- if infrarenal control appears to be difficult, a clamp can be placed on the suprarenal aorta
- the aneurysm should be opened only after proximal and distal control have been achieved
- once the proximal anastomosis is complete, the proximal clamp should be moved to the graft
- must take great care to avoid injury to the left renal vein, vena cava, iliac and lumbar veins
- Endovascular Repair
- some studies suggest that there is less morbidity and mortality for EVAR
- operative planning will require a CTA or angiogram
- the contraindications for EVAR for ruptured AAA are the same as for elective AAA repair
- procedure may be done under local anesthesia with sedation, and is essentially performed just
like an elective EVAR
Aortic Dissections
- Pathophysiology
- intimal tear creates a false channel within the aortic wall between the media and adventitia
- if the adventitia cannot contain the pressurized blood, aortic rupture and exsanguination occurs
- blood flow in the false lumen can occlude the branching vessels of the aorta, leading to a variety
of acute malperfusion syndromes
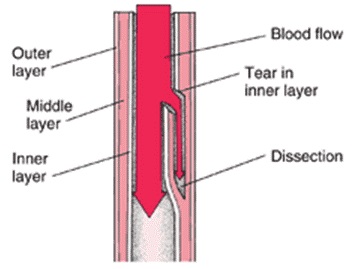
- Risk Factors
- hypertension
- connective tissue disorders (Marfan’s disease)
- pregnancy
- cocaine use
- Classification
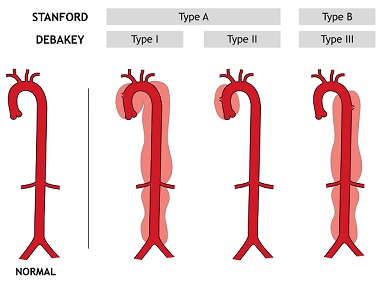
- DeBakey
- categorizes the dissection based on the origin of the intimal tear and the extent of the
dissection
- DeBakey type I: involves the ascending aorta, aortic arch, and the descending aorta
- DeBakey type II: involves only the ascending aorta
- DeBakey type III: originates distal to the left subclavian artery
- Stanford
- Stanford A: all dissections involving the ascending aorta, regardless of the site of origin
(DeBakey types I and II)
- Stanford B: do not involve the ascending aorta (DeBakey type III)
- Clinical Presentation
- acute, severe (10/10), tearing or ripping chest pain radiating to the back between the scapulae
- in type A dissections, pericardial tamponade or aortic valve disruption may be present
- hypoperfusion of the spine, renal, mesenteric, or lower extremity vessels may complicate both
type A and B dissections
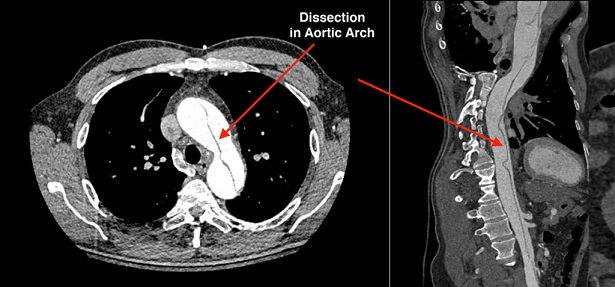
- Diagnosis
- CTA provides excellent anatomic data, visualizes the entry tear, allows assessment of branch vessel
patency, and detects rupture
- TEE is valuable for assessing for tamponade, valvular disruption, and cardiac wall motion abnormalities
- conventional aortography is rarely used in the acute setting
- Management
- an arterial line should be placed in the extremity with the best pulse
- beta blockers should be started immediately and titrated to a heart rate of 60 – 70 bpm and a
systolic blood pressure of 100 – 110 mm Hg
- Stanford Type A
- surgical emergency
- cardiopulmonary bypass is required
- ascending aorta must be replaced by a tube interposition graft, which reestablishes flow
into the true lumen
- Stanford Type B
- medical management is the preferred treatment for uncomplicated dissections
- for patients with complicated type B dissections, thoracic endovascular repair (TEVAR) has
largely replaced open surgery
References
- Schwartz, 10th ed., pgs 806 - 817, 850 - 859
- Sabiston, 20th ed., pgs 1722 - 1738, 1746 - 1750
- Cameron, 13th ed., pgs 901 – 905, 905 – 911, 911 – 915, 922 - 928
- UpToDate. Management of Asymptomatic Abdominal Aortic Aneurysm. Ronald L. Dalman, MD, Matthew Mell, MD, MS, FACS.
Jun 12, 2020. Pgs 1 – 70









